Effectiveness of smoking-cessation interventions for urban hospital patients: study protocol for a randomized controlled trial
- PMID: 22852878
- PMCID: PMC3502597
- DOI: 10.1186/1745-6215-13-126
Effectiveness of smoking-cessation interventions for urban hospital patients: study protocol for a randomized controlled trial
Abstract
Background: Hospitalization may be a particularly important time to promote smoking cessation, especially in the immediate post-discharge period. However, there are few studies to date that shed light on the most effective or cost-effective methods to provide post-discharge cessation treatment, especially among low-income populations and those with a heavy burden of mental illness and substance use disorders.
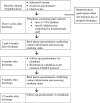
Methods/design: This randomized trial will compare the effectiveness and cost-effectiveness of two approaches to smoking cessation treatment among patients discharged from two urban public hospitals in New York City. During hospitalization, staff will be prompted to ask about smoking and to offer nicotine replacement therapy (NRT) on admission and at discharge. Subjects will be randomized on discharge to one of two arms: one arm will be proactive multi-session telephone counseling with motivational enhancement delivered by study staff, and the other will be a faxed or online referral to the New York State Quitline. The primary outcome is 30-day point-prevalence abstinence from smoking at 6-month follow-up post-discharge. We will also examine cost-effectiveness from a societal and a payer perspective, as well as explore subgroup analyses related to patient location of hospitalization, race/ethnicity, immigrant status, and inpatient diagnosis.
Discussion: This study will explore issues of implementation feasibility in a post-hospitalization patient population, as well as add information about the effectiveness and cost-effectiveness of different strategies for designing smoking cessation programs for hospitalized patients.
Trial registration: Clinicaltrials.gov ID# NCT01363245.
Figures
References
-
- Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults and trends in smoking cessation - United States. MMWR 2009. 2008;58:1227–1232. - PubMed
-
- Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged > or = 18 years - United States. MMWR 2010. 2009;59:1135–1140. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services Public Health Service; 2008.
-
- Rigotti NA, Munafo MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2007;18:CD001837. - PubMed
-
- Stead LF, Lancaster T, Perera R. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2003;1:CD002850. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical