Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries
- PMID: 22853001
- PMCID: PMC3454511
- DOI: 10.1021/jo300974j
Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries
Abstract
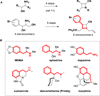
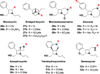
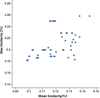
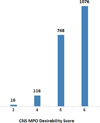
The synthesis and diversification of a densely functionalized azetidine ring system to gain access to a wide variety of fused, bridged, and spirocyclic ring systems is described. The in vitro physicochemical and pharmacokinetic properties of representative library members are measured in order to evaluate the use of these scaffolds for the generation of lead-like molecules to be used in targeting the central nervous system. The solid-phase synthesis of a 1976-membered library of spirocyclic azetidines is also described.
Figures


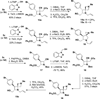
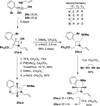

References
-
- Morton D, Leach S, Cordier C, Warriner S, Nelson A. Angew. Chem. Int. Ed. 2009;48:104–109. - PMC - PubMed
- Arya P, Joseph R, Gan Z, Rakic B. Chem. Biol. 2005;12:163–180. - PubMed
- Tan DS. Nat. Chem. Biol. 2005;1:74–84. - PubMed
- Burke MD, Schreiber SL. Angew. Chem. Int. Ed. 2004;43:46–58. - PubMed
- Schreiber SL. Science. 2000;287:1964–1969. - PubMed
-
- Tepper RI, Roubenoff R. In: Genomics and Personalized Medicine. Willard HF, Ginsburg GS, editors. Elsevier Inc.; 2009. pp. 335–342.
-
- Payne RH, Gwynn MN, Holmes DJ, Pompliano DL. Nat. Rev. Drug Disc. 2007;6:29–40. - PubMed
-
- Huryn DM, Jeffrey L, Brodsky JL, Brummond KM, Chambers PG, Eyer B, Ireland AW, Kawasumi M, LaPorte MG, Lloyd K, Manteau B, Nghiem P, Quade B, Seguin SP, Wipf P. Proc. Natl. Acad. Sci. 2011;108:6757–6762. - PMC - PubMed
- Cui J, Hao J, Ulanovskaya OA, Dundas J, Liang J, Kozmin SA. Proc. Natl. Acad. Sci. 2011;108:6763–6768. - PMC - PubMed
- Marcaurelle LA, Comer E, Dandapani S, Duvall JR, Gerard B, Kesavan S, Lee MD, IV, Liu H, Lowe JT, Marie J-C, Mulrooney CA, Pandya BA, Rowley A, Ryba TD, Suh B-C, Wei J, Young DW, Akella LB, Ross NT, Zhang Y-L, Fass DM, Reis SA, Zhao W-Z, Haggarty SJ, Palmer M, Foley MA. J. Am. Chem. Soc. 2010;132:16962–16976. - PMC - PubMed
- Bauer RA, DiBlasi CM, Tan DS. Org. Lett. 2010;12:2084–2087. - PMC - PubMed
- Diaz-Gavilan M, Galloway WRJD, O’Connell KMG, Hogkingson JT, Spring DR. Chem. Comm. 2010;46:776–778. - PubMed
- Oguri H, Schreiber SL. Org. Lett. 2005;7:47–50. - PubMed
- Boldi AM. Curr. Opin. Chem. Bio. 2004;8:281–286. - PubMed
- Taylor SJ, Taylor AM, Schreiber SL. Angew. Chem. Int. Ed. 2004;43:1681–1685. - PubMed
- Lo MMC, Neumann CS, Nagayama S, Perlstein EO, Schreiber SL. J. Am. Chem. Soc. 2004;126:16077–16086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

