Addition of granulocyte macrophage colony stimulating factor does not improve response to early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab
- PMID: 22853816
- PMCID: PMC4419690
- DOI: 10.3109/10428194.2012.717276
Addition of granulocyte macrophage colony stimulating factor does not improve response to early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab
Abstract
Thirty-three previously untreated patients with high-risk chronic lymphocytic leukemia (CLL) were treated before meeting standard criteria with alemtuzumab and rituximab. Granulocyte macrophage colony stimulating factor (GM-CSF) was added to the regimen to determine whether it would improve treatment efficacy without increasing toxicity. High risk was defined as at least one of the following: 17p13-; 11q22.3-; unmutated IGHV (or use of VH3-21) together with elevated expression of ZAP-70 and/or CD38. Treatment was subcutaneous GM-CSF 250 μg Monday-Wednesday-Friday for 6 weeks from day 1, subcutaneous alemtuzumab 3 mg-10 mg-30 mg from day 3 and then 30 mg Monday-Wednesday-Friday for 4 weeks, and intravenous rituximab (375 mg/m(2)/week) for 4 weeks from day 8. Patients received standard supportive care and were monitored weekly for cytomegalovirus (CMV) reactivation. Using standard criteria, 31 (94%) patients responded to treatment, with nine (27%) complete responses (one with persistent cytopenia) and nine (27%) nodular partial responses. Median progression-free survival was 13.0 months and time to next treatment was 33.5 months. No patient died during treatment, seven (21%) had grade 3-4 toxicities attributable to treatment, and 10 (30%) had CMV viremia. Addition of GM-CSF to therapy with alemtuzumab and rituximab decreased treatment efficacy and increased the rate of CMV reactivation compared to a historical control.
Trial registration: ClinicalTrials.gov NCT00562328.
Figures
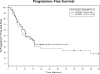
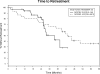

Comment in
-
"Primum non nocere": the addition of granulocyte-macrophage colony stimulating factor to alemtuzumab in chronic lymphocytic leukemia.Leuk Lymphoma. 2013 Mar;54(3):441-2. doi: 10.3109/10428194.2012.727420. Epub 2012 Sep 28. Leuk Lymphoma. 2013. PMID: 22950412 No abstract available.
References
-
- Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. - PubMed
-
- Hamblin T, Davis Z, Gardiner A, Oscier D, Stevenson F. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. - PubMed
-
- Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. - PubMed
-
- Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. - PubMed
-
- Tobin G, Thunberg U, Johnson A, et al. Chronic lymphocytic leukemias utilizing the VH3-21 gene display highly restricted Vlambda2-14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood. 2003;101:4952–4957. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
