Pharmacokinetic profiles of artesunate following multiple intravenous doses of 2, 4, and 8 mg/kg in healthy volunteers: phase 1b study
- PMID: 22853818
- PMCID: PMC3468400
- DOI: 10.1186/1475-2875-11-255
Pharmacokinetic profiles of artesunate following multiple intravenous doses of 2, 4, and 8 mg/kg in healthy volunteers: phase 1b study
Abstract
Background: Severe malaria results in over a million deaths every year, most of them in children aged less than five years and living in sub-Saharan Africa. Injectable artesunate (AS) was recommended as initial treatment for severe malaria by WHO in 2006. The Walter Reed Army Institute of Research (WRAIR) has been developing a novel good manufacturing practice (GMP) injection of AS, which was approved by the US FDA for investigational drug use and distribution by the CDC.
Methods: Tolerability and pharmacokinetics of current GMP intravenous AS, as an anti-malarial agent, were evaluated after ascending multiple doses of 2, 4, and 8 mg/kg daily for three days with 2-minute infusion in 24 healthy subjects (divided into three groups) in the Phase 1 clinical trial study.
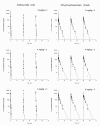
Results: Results showed that there were no dose-dependent increases in any adverse events. Drug concentrations showed no accumulation and no decline of the drug during the three days of treatment. After intravenous injection, parent drug rapidly declined and was converted to dihydroartemisinin (DHA) with overall mean elimination half-lives ranging 0.15-0.23 hr for AS and 1.23-1.63 hr for DHA, but the peak concentration (C(max)) of AS was much higher than that of DHA with a range of 3.08-3.78-folds. In addition, the AUC and C(max) values of AS and DHA were increased proportionally to the AS climbing multiple doses.
Discussion: The safety of injectable AS, even at the highest dose of 8 mg/kg increases the probability of therapeutic success of the drug even in patients with large variability of parasitaemia.
Figures

Similar articles
-
Pharmacokinetic evaluation of intravenous artesunate in adults with uncomplicated falciparum malaria in Kenya: a phase II study.Malar J. 2014 Jul 22;13:281. doi: 10.1186/1475-2875-13-281. Malar J. 2014. PMID: 25047305 Free PMC article. Clinical Trial.
-
Pharmacokinetic profiles of artesunate after single intravenous doses at 0.5, 1, 2, 4, and 8 mg/kg in healthy volunteers: a phase I study.Am J Trop Med Hyg. 2009 Oct;81(4):615-21. doi: 10.4269/ajtmh.2009.09-0150. Am J Trop Med Hyg. 2009. PMID: 19815876 Clinical Trial.
-
Population pharmacokinetics of artesunate and dihydroartemisinin following intra-rectal dosing of artesunate in malaria patients.PLoS Med. 2006 Nov;3(11):e444. doi: 10.1371/journal.pmed.0030444. PLoS Med. 2006. PMID: 17132053 Free PMC article.
-
Systematic review of artesunate pharmacokinetics: Implication for treatment of resistant malaria.Int J Infect Dis. 2019 Dec;89:30-44. doi: 10.1016/j.ijid.2019.08.030. Epub 2019 Sep 3. Int J Infect Dis. 2019. PMID: 31491558
-
Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration.Malar J. 2011 Sep 13;10:263. doi: 10.1186/1475-2875-10-263. Malar J. 2011. PMID: 21914160 Free PMC article. Review.
Cited by
-
Safety and efficacy of artesunate treatment in severely injured patients with traumatic hemorrhage. The TOP-ART randomized clinical trial.Intensive Care Med. 2023 Aug;49(8):922-933. doi: 10.1007/s00134-023-07135-3. Epub 2023 Jul 20. Intensive Care Med. 2023. PMID: 37470832 Free PMC article. Clinical Trial.
-
Artesunate overcomes drug resistance in multiple myeloma by inducing mitochondrial stress and non-caspase apoptosis.Oncotarget. 2014 Jun 30;5(12):4118-28. doi: 10.18632/oncotarget.1847. Oncotarget. 2014. PMID: 24948357 Free PMC article.
-
Predicting the Disposition of the Antimalarial Drug Artesunate and Its Active Metabolite Dihydroartemisinin Using Physiologically Based Pharmacokinetic Modeling.Antimicrob Agents Chemother. 2021 Feb 17;65(3):e02280-20. doi: 10.1128/AAC.02280-20. Print 2021 Feb 17. Antimicrob Agents Chemother. 2021. PMID: 33361307 Free PMC article.
-
Investigating the Comparative Effects of Six Artemisinin-based Combination Therapies on Plasmodium-induced Hepatorenal Toxicity.Niger Med J. 2019 Jul-Aug;60(4):211-218. doi: 10.4103/nmj.NMJ_152_18. Epub 2019 Nov 25. Niger Med J. 2019. PMID: 31831942 Free PMC article.
-
Artesunate shows potent anti-tumor activity in B-cell lymphoma.J Hematol Oncol. 2018 Feb 20;11(1):23. doi: 10.1186/s13045-018-0561-0. J Hematol Oncol. 2018. PMID: 29458389 Free PMC article.
References
-
- Li Q, Gerena L, Xie L, Zhang J, Kyle D, Milhous W. Development and validation of flow cytometric measurement for parasitemia in cultures of P. falciparum vitally stained with YOYO-1. Cytometry A. 2007;71:297–307. - PubMed
-
- Li Q, Mog SR, Si YZ, Kyle DE, Gettayacamin M, Milhous WK. Neurotoxicity and efficacy of arteether related to its exposure times and exposure levels in rodents. Am J Trop Med Hyg. 2002;66:516–525. - PubMed
-
- McLean WG, Ward SA. In vitro neurotoxicity of artemisinin derivatives. Med Trop (Mars) 1998;58(3 Suppl):28–31. - PubMed
-
- Byakika-Kibwika P, Lamorde M, Mayito J, Nabukeera L, Mayanja-Kizza H, Katabira E, Hanpithakpong W, Obua C, Pakker N, Lindegardh N, Tarning J, de Vries PJ, Merry C. Pharmacokinetics and pharmacodynamics of intravenous artesunate during severe malaria treatment in Ugandan adults. Malar J. 2012;11:132. doi: 10.1186/1475-2875-11-132. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

