Sensing and responding to membrane tension: the bacterial MscL channel as a model system
- PMID: 22853893
- PMCID: PMC3400780
- DOI: 10.1016/j.bpj.2012.06.021
Sensing and responding to membrane tension: the bacterial MscL channel as a model system
Abstract
Mechanosensors are important for many life functions, including the senses of touch, balance, and proprioception; cardiovascular regulation; kidney function; and osmoregulation. Many channels from an assortment of families are now candidates for eukaryotic mechanosensors and proprioception, as well as cardiovascular regulation, kidney function, and osmoregulation. Bacteria also possess two families of mechanosensitive channels, termed MscL and MscS, that function as osmotic emergency release valves. Of the two channels, MscL is the most conserved, most streamlined in structure, and largest in conductance at 3.6 nS with a pore diameter in excess of 30 Å; hence, the structural changes required for gating are exaggerated and perhaps more easily defined. Because of these properties, as well as its tractable nature, MscL represents a excellent model for studying how a channel can sense and respond to biophysical changes of a lipid bilayer. Many of the properties of the MscL channel, such as the sensitivity to amphipaths, a helix that runs along the membrane surface and is connected to the pore via a glycine, a twisting and turning of the transmembrane domains upon gating, and the dynamic changes in membrane interactions, may be common to other candidate mechanosensors. Here we review many of these properties and discuss their structural and functional implications.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
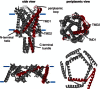
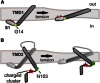
References
-
- Blount P., Li Y., Iscla I. Mechanosensitive channels gated by membrane tension: bacteria and beyond. In: Kamkin A., Kiseleva I., editors. Mechanosensitive Ion Channels. Springer Press; New York: 2008. pp. 71–101.
-
- Kumánovics A., Levin G., Blount P. Family ties of gated pores: evolution of the sensor module. FASEB J. 2002;16:1623–1629. - PubMed
-
- Martinac B., Adler J., Kung C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 1990;348:261–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

