Population shift between the open and closed states changes the water permeability of an Aquaporin Z mutant
- PMID: 22853898
- PMCID: PMC3400767
- DOI: 10.1016/j.bpj.2012.05.049
Population shift between the open and closed states changes the water permeability of an Aquaporin Z mutant
Abstract
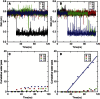
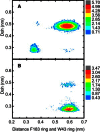
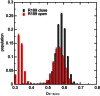
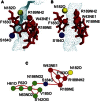
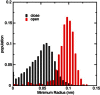
Aquaporins are tetrameric transmembrane channels permeable to water and other small solutes. Wild-type (WT) and mutant Aquaporin Z (AqpZ) have been widely studied and multiple factors have been found to affect their water permeability. In this study, molecular dynamics simulations have been performed for the tetrameric AqpZ F43W/H174G/T183F mutant. It displayed ∼10% average water permeability compared to WT AqpZ, which had been attributed to the increased channel lumen hydrophobicity. Our simulations, however, show a ring stacking between W43 and F183 acting as a secondary steric gate in the triple mutant with R189 as the primary steric gate in both mutant and WT AqpZ. The double gates (R189 and W43-F183) result in a high population of the closed conformation in the mutant. Occasionally an open state, with diffusive water permeability very close to that of WT AqpZ, was observed. Taken together, our results show that the double-gate mechanism is sufficient to explain the reduced water permeability in the mutant without invoking effects arising from increased hydrophobicity of the channel lumen. Our findings provide insights into how aquaporin-mediated water transport can be modulated and may further point to how aquaporin function can be optimized for biomimetic membrane applications.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Water permeation dynamics of AqpZ: a tale of two states.Biochim Biophys Acta. 2011 Jun;1808(6):1581-6. doi: 10.1016/j.bbamem.2011.02.001. Epub 2011 Feb 18. Biochim Biophys Acta. 2011. PMID: 21315687
-
Functional reconstitution and characterization of AqpZ, the E. coli water channel protein.J Mol Biol. 1999 Sep 3;291(5):1169-79. doi: 10.1006/jmbi.1999.3032. J Mol Biol. 1999. PMID: 10518952
-
Highly selective water channel activity measured by voltage clamp: analysis of planar lipid bilayers reconstituted with purified AqpZ.Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9624-9. doi: 10.1073/pnas.161299398. Epub 2001 Aug 7. Proc Natl Acad Sci U S A. 2001. PMID: 11493683 Free PMC article.
-
Dynamics and energetics of permeation through aquaporins. What do we learn from molecular dynamics simulations?Handb Exp Pharmacol. 2009;(190):57-76. doi: 10.1007/978-3-540-79885-9_3. Handb Exp Pharmacol. 2009. PMID: 19096772 Review.
-
Experimental and Simulation Studies of Aquaporin 0 Water Permeability and Regulation.Chem Rev. 2019 May 8;119(9):6015-6039. doi: 10.1021/acs.chemrev.9b00106. Epub 2019 Apr 26. Chem Rev. 2019. PMID: 31026155 Review.
Cited by
-
Optimization of Aquaporin Loading for Performance Enhancement of Aquaporin-Based Biomimetic Thin-Film Composite Membranes.Membranes (Basel). 2021 Dec 27;12(1):32. doi: 10.3390/membranes12010032. Membranes (Basel). 2021. PMID: 35054558 Free PMC article.
-
Increasing Salt Rejection of Polybenzimidazole Nanofiltration Membranes via the Addition of Immobilized and Aligned Aquaporins.Processes (Basel). 2019 Feb;7(2):76. doi: 10.3390/pr7020076. Epub 2019 Feb 3. Processes (Basel). 2019. PMID: 31179235 Free PMC article.
-
Coupled Mutations-Enabled Glycerol Transportation in an Aquaporin Z Mutant.ACS Omega. 2018 Apr 12;3(4):4113-4122. doi: 10.1021/acsomega.8b00126. eCollection 2018 Apr 30. ACS Omega. 2018. PMID: 31458647 Free PMC article.
References
-
- Borgnia M., Nielsen S., Agre P. Cellular and molecular biology of the aquaporin water channels. Annu. Rev. Biochem. 1999;68:425–458. - PubMed
-
- King L.S., Kozono D., Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004;5:687–698. - PubMed
-
- Murata K., Mitsuoka K., Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. - PubMed
-
- Carbrey J.M., Agre P. Discovery of the aquaporins and development of the field. Handb. Exp. Pharmacol. 2009:3–28. - PubMed
-
- Nielsen C.H. Major intrinsic proteins in biomimetic membranes. Adv. Exp. Med. Biol. 2010;679:127–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

