Insights from free-energy calculations: protein conformational equilibrium, driving forces, and ligand-binding modes
- PMID: 22853912
- PMCID: PMC3400776
- DOI: 10.1016/j.bpj.2012.05.046
Insights from free-energy calculations: protein conformational equilibrium, driving forces, and ligand-binding modes
Abstract
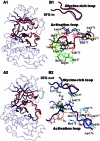
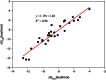
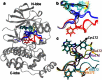
Accurate free-energy calculations provide mechanistic insights into molecular recognition and conformational equilibrium. In this work, we performed free-energy calculations to study the thermodynamic properties of different states of molecular systems in their equilibrium basin, and obtained accurate absolute binding free-energy calculations for protein-ligand binding using a newly developed M2 algorithm. We used a range of Asp-Phe-Gly (DFG)-in/out p38α mitogen-activated protein kinase inhibitors as our test cases. We also focused on the flexible DFG motif, which is closely connected to kinase activation and inhibitor binding. Our calculations explain the coexistence of DFG-in and DFG-out states of the loop and reveal different components (e.g., configurational entropy and enthalpy) that stabilize the apo p38α conformations. To study novel ligand-binding modes and the key driving forces behind them, we computed the absolute binding free energies of 30 p38α inhibitors, including analogs with unavailable experimental structures. The calculations revealed multiple stable, complex conformations and changes in p38α and inhibitor conformations, as well as balance in several energetic terms and configurational entropy loss. The results provide relevant physics that can aid in designing inhibitors and understanding protein conformational equilibrium. Our approach is fast for use with proteins that contain flexible regions for structure-based drug design.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
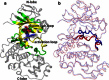

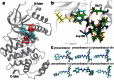
References
-
- Huse M., Kuriyan J. The conformational plasticity of protein kinases. Cell. 2002;109:275–282. - PubMed
-
- Bert K., Gerhard M., Michael H., editors. Protein Kinases as Drug Targets. Wiley-VCH; Weinheim: 2011.
-
- Vogtherr M., Saxena K., Schwalbe H. NMR characterization of kinase p38 dynamics in free and ligand-bound forms. Angew. Chem. Int. Ed. Engl. 2006;45:993–997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

