Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes
- PMID: 22854038
- PMCID: PMC3533391
- DOI: 10.1242/jcs.105015
Polo-like kinase is required for synaptonemal complex disassembly and phosphorylation in mouse spermatocytes
Abstract
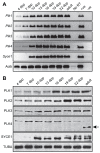
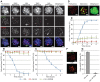
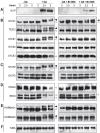
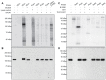
During meiosis, accurate coordination of the completion of homologous recombination and synaptonemal complex (SC) disassembly during the prophase to metaphase I (G2/MI) transition is essential to avoid aneuploid gametes and infertility. Previous studies have shown that kinase activity is required to promote meiotic prophase exit. The first step of the G2/MI transition is the disassembly of the central element components of the SC; however, the kinase(s) required to trigger this process remains unknown. Here we assess roles of polo-like kinases (PLKs) in mouse spermatocytes, both in vivo and during prophase exit induced ex vivo by the phosphatase inhibitor okadaic acid. All four PLKs are expressed during the first wave of spermatogenesis. Only PLK1 (not PLK2-4) localizes to the SC during the G2/MI transition. The SC central element proteins SYCP1, TEX12 and SYCE1 are phosphorylated during the G2/MI transition. However, treatment of pachytene spermatocytes with the PLK inhibitor BI 2536 prevented the okadaic-acid-induced meiotic prophase exit and inhibited phosphorylation of the central element proteins as well as their removal from the SC. Phosphorylation assays in vitro demonstrated that PLK1, but not PLK2-4, phosphorylates central element proteins SYCP1 and TEX12. These findings provide mechanistic details of the first stage of SC disassembly in mammalian spermatocytes, and reveal that PLK-mediated phosphorylation of central element proteins is required for meiotic prophase exit.
Figures


References
-
- Alexander J., Lim D., Joughin B. A., Hegemann B., Hutchins J. R. A., Ehrenberger T., Ivins F., Sessa F., Hudecz O., Nigg E. A.et al. (2011). Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling. Sci. Signal. 4, ra42 10.1126/scisignal.2001796 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

