Defining the mode of tumour growth by clonal analysis
- PMID: 22854777
- PMCID: PMC5553110
- DOI: 10.1038/nature11344
Defining the mode of tumour growth by clonal analysis
Abstract
Recent studies using the isolation of a subpopulation of tumour cells followed by their transplantation into immunodeficient mice provide evidence that certain tumours, including squamous skin tumours, contain cells with high clonogenic potential that have been referred to as cancer stem cells (CSCs). Until now, CSC properties have only been investigated by transplantation assays, and their existence in unperturbed tumour growth is unproven. Here we make use of clonal analysis of squamous skin tumours using genetic lineage tracing to unravel the mode of tumour growth in vivo in its native environment. To this end, we used a genetic labelling strategy that allows individual tumour cells to be marked and traced over time at different stages of tumour progression. Surprisingly, we found that the majority of labelled tumour cells in benign papilloma have only limited proliferative potential, whereas a fraction has the capacity to persist long term, giving rise to progeny that occupy a significant part of the tumour. As well as confirming the presence of two distinct proliferative cell compartments within the papilloma, mirroring the composition, hierarchy and fate behaviour of normal tissue, quantitative analysis of clonal fate data indicates that the more persistent population has stem-cell-like characteristics and cycles twice per day, whereas the second represents a slower cycling transient population that gives rise to terminally differentiated tumour cells. Such behaviour is shown to be consistent with double-labelling experiments and detailed clonal fate characteristics. By contrast, measurements of clone size and proliferative potential in invasive squamous cell carcinoma show a different pattern of behaviour, consistent with geometric expansion of a single CSC population with limited potential for terminal differentiation. This study presents the first experimental evidence for the existence of CSCs during unperturbed solid tumour growth.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


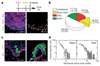
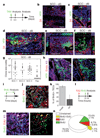
Comment in
-
Tumour evolution: Evidence points to the existence of cancer stem cells.Nat Rev Clin Oncol. 2012 Oct;9(10):552. doi: 10.1038/nrclinonc.2012.149. Epub 2012 Aug 14. Nat Rev Clin Oncol. 2012. PMID: 22889975 No abstract available.
-
Cancer stem cells: Tracing clones.Nat Rev Cancer. 2012 Sep;12(9):579. doi: 10.1038/nrc3354. Epub 2012 Aug 17. Nat Rev Cancer. 2012. PMID: 22898540 No abstract available.
-
Cancer: Resolving the stem-cell debate.Nature. 2012 Aug 23;488(7412):462-3. doi: 10.1038/nature11480. Nature. 2012. PMID: 22919708 No abstract available.
-
The root of all evil.Nat Methods. 2012 Oct;9(10):942-3. doi: 10.1038/nmeth.2188. Nat Methods. 2012. PMID: 23193564 No abstract available.
References
-
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. - PubMed
-
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. - PubMed
-
- Malanchi I, et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature. 2008;452:650–653. - PubMed
-
- Beck B, et al. A vascular niche and a VEGF–Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

