Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide
- PMID: 22854889
- PMCID: PMC3576922
- DOI: 10.1007/s00125-012-2649-3
Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide
Abstract
Aims/hypothesis: Plasma ceramide concentrations correlate with insulin sensitivity, inflammation and atherosclerotic risk. We hypothesised that plasma ceramide concentrations are increased in the presence of elevated fatty acid levels and are regulated by increased liver serine C-palmitoyltransferase (SPT) activity.
Methods: Lean humans and rats underwent an acute lipid infusion and plasma ceramide levels were determined. One group of lipid-infused rats was administered myriocin to inhibit SPT activity. Liver SPT activity was determined in lipid-infused rats, and obese, insulin resistant mice. The time and palmitate dose-dependent synthesis of intracellular and secreted ceramide was determined in HepG2 liver cells.
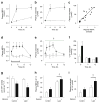
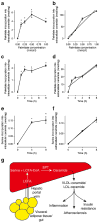
Results: Plasma ceramide levels were increased during lipid infusion in humans and rats, and in obese, insulin-resistant mice. The increase in plasma ceramide was not associated with changes in liver SPT activity, and inhibiting SPT activity by ~50% did not alter plasma ceramide levels in lipid-infused rats. In HepG2 liver cells, palmitate incorporation into extracellular ceramide was both dose- and time-dependent, suggesting the liver cells rapidly secreted the newly synthesised ceramide.
Conclusions/interpretation: Elevated systemic fatty acid availability increased plasma ceramide but this was not associated with changes in hepatic SPT activity, suggesting that liver ceramide synthesis is driven by substrate availability rather than increased SPT activity. This report also provides evidence that the liver is sensitive to the intracellular ceramide concentration, and an increase in liver ceramide secretion may help protect the liver from the deleterious effects of intracellular ceramide accumulation.
Conflict of interest statement
Figures
References
-
- Lipina C, Hundal HS. Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance. Diabetologia. 2011;54:1596–1607. - PubMed
-
- de Mello VD, Lankinen M, Schwab U, et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. - PubMed
-
- Ichi I, Nakahara K, Miyashita Y, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. - PubMed
-
- Wiesner P, Leidl K, Boettcher A, Schmitz G, Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

