Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance
- PMID: 22854958
- PMCID: PMC3460483
- DOI: 10.1074/jbc.M111.309666
Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance
Abstract
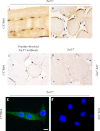
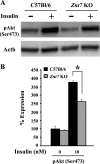
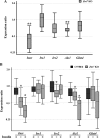
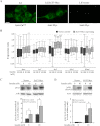
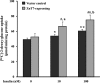
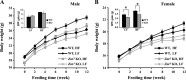
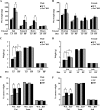
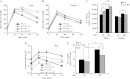
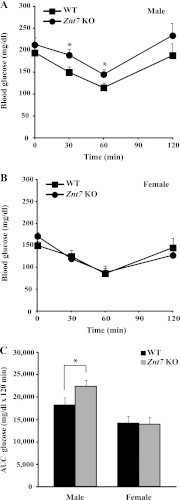
The Znt7 gene encodes a ubiquitously expressed zinc transporter that is involved in transporting cytoplasmic zinc into the Golgi apparatus and a ZnT7-containing vesicular compartment. Overexpression of ZnT7 in the pancreatic β-cell stimulates insulin synthesis and secretion through regulation of insulin gene transcription. In this study, we demonstrate that ZnT7 is expressed in the mouse skeletal muscle. The activity of the insulin signaling pathway was down-regulated in myocytes isolated from the femoral muscle of Znt7 knock-out (KO) mice. High fat diet consumption (45% kcal) induced weight gain in male Znt7 KO mice but not female Znt7 KO mice. Male Znt7 KO mice fed the high fat diet at 5 weeks of age for 10 weeks exhibited hyperglycemia in the non-fasting state. Oral glucose tolerance tests revealed that male Znt7 KO mice fed the high fat diet had severe glucose intolerance. Insulin tolerance tests showed that male Znt7 KO mice were insulin-resistant. Diet-induced insulin resistance in male Znt7 KO mice was paralleled by a reduction in mRNA expression of Insr, Irs2, and Akt1 in the primary skeletal myotubes isolated from the KO mice. Overexpression of ZnT7 in a rat skeletal muscle cell line (L6) increased Irs2 mRNA expression, Irs2 and Akt phosphorylation, and glucose uptake. We conclude that a combination of decreased insulin secretion and increased insulin resistance accounts for the glucose intolerance observed in Znt7 KO mice.
Figures
Similar articles
-
The Znt7-null mutation has sex dependent effects on the gut microbiota and goblet cell population in the mouse colon.PLoS One. 2020 Sep 29;15(9):e0239681. doi: 10.1371/journal.pone.0239681. eCollection 2020. PLoS One. 2020. PMID: 32991615 Free PMC article.
-
Aberrant fatty acid metabolism in skeletal muscle contributes to insulin resistance in zinc transporter 7 (znt7)-knockout mice.J Biol Chem. 2018 May 18;293(20):7549-7563. doi: 10.1074/jbc.M117.817692. Epub 2018 Mar 19. J Biol Chem. 2018. PMID: 29555680 Free PMC article.
-
Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake.FEBS J. 2016 Jan;283(2):378-94. doi: 10.1111/febs.13582. Epub 2015 Dec 12. FEBS J. 2016. PMID: 26524605
-
Subcongenic analysis of a quantitative trait locus affecting body weight and glucose metabolism in zinc transporter 7 (znt7)-knockout mice.BMC Genet. 2019 Feb 18;20(1):19. doi: 10.1186/s12863-019-0715-2. BMC Genet. 2019. PMID: 30777014 Free PMC article.
-
Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic beta-cells by promoting insulin gene transcription.Exp Cell Res. 2010 Oct 1;316(16):2630-43. doi: 10.1016/j.yexcr.2010.06.017. Epub 2010 Jul 1. Exp Cell Res. 2010. PMID: 20599947
Cited by
-
The Znt7-null mutation has sex dependent effects on the gut microbiota and goblet cell population in the mouse colon.PLoS One. 2020 Sep 29;15(9):e0239681. doi: 10.1371/journal.pone.0239681. eCollection 2020. PLoS One. 2020. PMID: 32991615 Free PMC article.
-
Effect of increasing zinc supplementation on post-transit performance, behavior, blood and muscle metabolites, and gene expression in growing beef feedlot steers.J Anim Sci. 2022 Sep 1;100(9):skac246. doi: 10.1093/jas/skac246. J Anim Sci. 2022. PMID: 35917831 Free PMC article.
-
Zinc at the crossroads of exercise and proteostasis.Redox Biol. 2020 Aug;35:101529. doi: 10.1016/j.redox.2020.101529. Epub 2020 Apr 1. Redox Biol. 2020. PMID: 32273258 Free PMC article. Review.
-
Zinc transporter 8 (ZnT8) and β cell function.Trends Endocrinol Metab. 2014 Aug;25(8):415-24. doi: 10.1016/j.tem.2014.03.008. Epub 2014 Apr 18. Trends Endocrinol Metab. 2014. PMID: 24751356 Free PMC article. Review.
-
SLC30A family expression in the pancreatic islets of humans and mice: cellular localization in the β-cells.J Mol Histol. 2018 Apr;49(2):133-145. doi: 10.1007/s10735-017-9753-0. Epub 2018 Jan 25. J Mol Histol. 2018. PMID: 29372370
References
-
- Palmiter R. D., Huang L. (2004) Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 447, 744–751 - PubMed
-
- Kambe T., Narita H., Yamaguchi-Iwai Y., Hirose J., Amano T., Sugiura N., Sasaki R., Mori K., Iwanaga T., Nagao M. (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J. Biol. Chem. 277, 19049–19055 - PubMed
-
- Jackson K. A., Helston R. M., McKay J. A., O'Neill E. D., Mathers J. C., Ford D. (2007) Splice variants of the human zinc transporter ZnT5 (SLC30A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J. Biol. Chem. 282, 10423–10431 - PubMed
-
- Eide D.J. (2004) The SLC39 family of metal ion transporters. Pflugers Arch. 447, 796–800 - PubMed
-
- Valentine R. A., Jackson K. A., Christie G. R., Mathers J. C., Taylor P. M., Ford D. (2007) ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 282, 14389–14393 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

