The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter
- PMID: 22854968
- PMCID: PMC3464577
- DOI: 10.1074/jbc.M112.384560
The deactive form of respiratory complex I from mammalian mitochondria is a Na+/H+ antiporter
Abstract
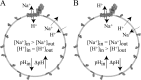
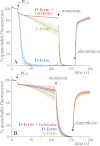
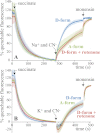
In mitochondria, complex I (NADH:ubiquinone oxidoreductase) uses the redox potential energy from NADH oxidation by ubiquinone to transport protons across the inner membrane, contributing to the proton-motive force. However, in some prokaryotes, complex I may transport sodium ions instead, and three subunits in the membrane domain of complex I are closely related to subunits from the Mrp family of Na(+)/H(+) antiporters. Here, we define the relationship between complex I from Bos taurus heart mitochondria, a close model for the human enzyme, and sodium ion transport across the mitochondrial inner membrane. In accord with current consensus, we exclude the possibility of redox-coupled Na(+) transport by B. taurus complex I. Instead, we show that the "deactive" form of complex I, which is formed spontaneously when enzyme turnover is precluded by lack of substrates, is a Na(+)/H(+) antiporter. The antiporter activity is abolished upon reactivation by the addition of substrates and by the complex I inhibitor rotenone. It is specific for Na(+) over K(+), and it is not exhibited by complex I from the yeast Yarrowia lipolytica, which thus has a less extensive deactive transition. We propose that the functional connection between the redox and transporter modules of complex I is broken in the deactive state, allowing the transport module to assert its independent properties. The deactive state of complex I is formed during hypoxia, when respiratory chain turnover is slowed, and may contribute to determining the outcome of ischemia-reperfusion injury.
Figures



References
-
- Hirst J. (2010) Toward the molecular mechanism of respiratory complex I. Biochem. J. 425, 327–339 - PubMed
-
- Efremov R. G., Sazanov L. A. (2011) Structure of the membrane domain of respiratory complex I. Nature 476, 414–420 - PubMed
-
- Mathiesen C., Hägerhäll C. (2002) Transmembrane topology of the NuoL, M, and N subunits of NADH:quinone oxidoreductase and their homologues among membrane-bound hydrogenases and bona fide antiporters. Biochim. Biophys. Acta 1556, 121–132 - PubMed
-
- Moparthi V. K., Kumar B., Mathiesen C., Hägerhäll C. (2011) Homologous protein subunits from Escherichia coli NADH:quinone oxidoreductase can functionally replace MrpA and MrpD in Bacillus subtilis. Biochim. Biophys. Acta 1807, 427–436 - PubMed
-
- Krah A., Pogoryelov D., Langer J. D., Bond P. J., Meier T., Faraldo-Gómez J. D. (2010) Structural and energetic basis for H+ versus Na+ binding selectivity in ATP synthase F0 rotors. Biochim. Biophys. Acta 1797, 763–772 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

