Distinctive features of catalytic and transport mechanisms in mammalian sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) and Cu+ (ATP7A/B) ATPases
- PMID: 22854969
- PMCID: PMC3463298
- DOI: 10.1074/jbc.M112.373472
Distinctive features of catalytic and transport mechanisms in mammalian sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) and Cu+ (ATP7A/B) ATPases
Abstract
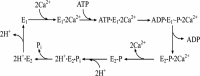
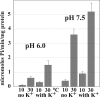
Ca(2+) (sarco-endoplasmic reticulum Ca(2+) ATPase (SERCA)) and Cu(+) (ATP7A/B) ATPases utilize ATP through formation of a phosphoenzyme intermediate (E-P) whereby phosphorylation potential affects affinity and orientation of bound cation. SERCA E-P formation is rate-limited by enzyme activation by Ca(2+), demonstrated by the addition of ATP and Ca(2+) to SERCA deprived of Ca(2+) (E2) as compared with ATP to Ca(2+)-activated enzyme (E1·2Ca(2+)). Activation by Ca(2+) is slower at low pH (2H(+)·E2 to E1·2Ca(2+)) and little sensitive to temperature-dependent activation energy. On the other hand, subsequent (forward or reverse) phosphoenzyme processing is sensitive to activation energy, which relieves conformational constraints limiting Ca(2+) translocation. A "H(+)-gated pathway," demonstrated by experiments on pH variations, charge transfer, and Glu-309 mutation allows luminal Ca(2+) release by H(+)/Ca(2+) exchange. As compared with SERCA, initial utilization of ATP by ATP7A/B is much slower and highly sensitive to temperature-dependent activation energy, suggesting conformational constraints of the headpiece domains. Contrary to SERCA, ATP7B phosphoenzyme cleavage shows much lower temperature dependence than EP formation. ATP-dependent charge transfer in ATP7A and -B is observed, with no variation of net charge upon pH changes and no evidence of Cu(+)/H(+) exchange. As opposed to SERCA after Ca(2+) chelation, ATP7A/B does not undergo reverse phosphorylation with P(i) after copper chelation unless a large N-metal binding extension segment is deleted. This is attributed to the inactivating interaction of the copper-deprived N-metal binding extension with the headpiece domains. We conclude that in addition to common (P-type) phosphoenzyme intermediate formation, SERCA and ATP7A/B possess distinctive features of catalytic and transport mechanisms.
Figures








References
-
- Albers R. W. (1967) Biochemical aspects of active transport. Annu. Rev. Biochem. 36, 727–756 - PubMed
-
- de Meis L., Vianna A. L. (1979) Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 48, 275–292 - PubMed
-
- Post R. L., Hegyvary C., Kume S. (1972) Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247, 6530–6540 - PubMed
-
- Brini M., Carafoli E. (2009) Calcium pumps in health and disease. Physiol. Rev. 89, 1341–1378 - PubMed
-
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. (1985) Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature 316, 696–700 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

