Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions
- PMID: 22855531
- PMCID: PMC3442403
- DOI: 10.1091/mbc.E12-02-0162
Nonredundant roles of cytoplasmic β- and γ-actin isoforms in regulation of epithelial apical junctions
Abstract
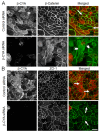
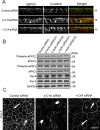
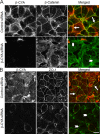
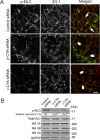
Association with the actin cytoskeleton is critical for normal architecture and dynamics of epithelial tight junctions (TJs) and adherens junctions (AJs). Epithelial cells express β-cytoplasmic (β-CYA) and γ-cytoplasmic (γ-CYA) actins, which have different cellular localization and functions. This study elucidates the roles of cytoplasmic actins in regulating structure and remodeling of AJs and TJs in model intestinal epithelia. Immunofluorescence labeling and latrunculin B treatment reveal affiliation of dynamic β-CYA filaments with newly assembled and mature AJs, whereas an apical γ-CYA pool is composed of stable perijunctional bundles and rapidly turning-over nonjunctional filaments. The functional effects of cytoplasmic actins on epithelial junctions are examined by using isoform-specific small interfering RNAs and cell-permeable inhibitory peptides. These experiments demonstrate unique roles of β-CYA and γ-CYA in regulating the steady-state integrity of AJs and TJs, respectively. Furthermore, β-CYA is selectively involved in establishment of apicobasal cell polarity. Both actin isoforms are essential for normal barrier function of epithelial monolayers, rapid AJ/TJ reassembly, and formation of three-dimensional cysts. Cytoplasmic actin isoforms play unique roles in regulating structure and permeability of epithelial junctions.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

