Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury
- PMID: 22855711
- PMCID: PMC3433235
- DOI: 10.4049/jimmunol.1200999
Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury
Abstract
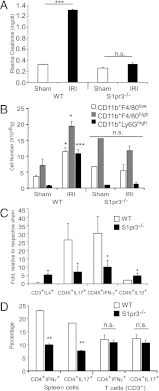
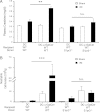
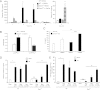
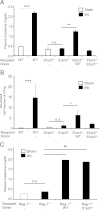
Dendritic cells (DCs) are central to innate and adaptive immunity of early kidney ischemia-reperfusion injury (IRI), and strategies to alter DC function may provide new therapeutic opportunities. Sphingosine 1-phosphate (S1P) modulates immunity through binding to its receptors (S1P1-5), and protection from kidney IRI occurs in S1P3-deficient mice. Through a series of experiments we determined that this protective effect was owing in part to differences between S1P3-sufficient and -deficient DCs. Mice lacking S1P3 on bone marrow cells were protected from IRI, and S1P3-deficient DCs displayed an immature phenotype. Wild-type (WT) but not S1P3-deficient DCs injected into mice depleted of DCs prior to kidney IR reconstituted injury. Adoptive transfer (i.e., i.v. injection) of glycolipid (Ag)-loaded WT but not S1P3-deficient DCs into WT mice exacerbated IRI, suggesting that WT but not S1P3-deficient DCs activated NKT cells. Whereas WT DC transfers activated the Th1/IFN-γ pathway, S1P3-deficient DCs activated the Th2/IL-4 pathway, and an IL-4-blocking Ab reversed protection from IRI, supporting the concept that IL-4 mediates the protective effect of S1P3-deficient DCs. Administration of S1P3-deficient DCs 7 d prior to or 3 h after IRI protected mice from IRI and suggests their potential use in cell-based therapy. We conclude that absence of DC S1P3 prevents DC maturation and promotes a Th2/IL-4 response. These findings highlight the importance of DC S1P3 in modulating NKT cell function and IRI and support development of selective S1P3 antagonists for tolerizing DCs for cell-based therapy or for systemic administration for the prevention and treatment of IRI and autoimmune diseases.
Figures



References
-
- Nelson P. J. 2007. Renal ischemia-reperfusion injury: renal dendritic cells loudly sound the alarm. Kidney Int. 71: 604–605 - PubMed
-
- Soos T. J., Sims T. N., Barisoni L., Lin K., Littman D. R., Dustin M. L., Nelson P. J. 2006. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 70: 591–596 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

