Traveling waves on the organ of corti of the chinchilla cochlea: spatial trajectories of inner hair cell depolarization inferred from responses of auditory-nerve fibers
- PMID: 22855802
- PMCID: PMC3436599
- DOI: 10.1523/JNEUROSCI.1138-12.2012
Traveling waves on the organ of corti of the chinchilla cochlea: spatial trajectories of inner hair cell depolarization inferred from responses of auditory-nerve fibers
Abstract
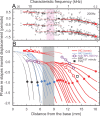
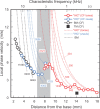
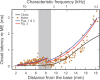
Spatial magnitude and phase profiles for inner hair cell (IHC) depolarization throughout the chinchilla cochlea were inferred from responses of auditory-nerve fibers (ANFs) to threshold- and moderate-level tones and tone complexes. Firing-rate profiles for frequencies ≤2 kHz are bimodal, with the major peak at the characteristic place and a secondary peak at 3-5 mm from the extreme base. Response-phase trajectories are synchronous with peak outward stapes displacement at the extreme cochlear base and accumulate 1.5 period lags at the characteristic places. High-frequency phase trajectories are very similar to the trajectories of basilar-membrane peak velocity toward scala tympani. Low-frequency phase trajectories undergo a polarity flip in a region, 6.5-9 mm from the cochlear base, where traveling-wave phase velocity attains a local minimum and a local maximum and where the onset latencies of near-threshold impulse responses computed from responses to near-threshold white noise exhibit a local minimum. That region is the same where frequency-threshold tuning curves of ANFs undergo a shape transition. Since depolarization of IHCs presumably indicates the mechanical stimulus to their stereocilia, the present results suggest that distinct low-frequency forward waves of organ of Corti vibration are launched simultaneously at the extreme base of the cochlea and at the 6.5-9 mm transition region, from where antiphasic reflections arise.
Figures


References
-
- Cai H, Temchin AN, Ruggero MA. Phase-frequency functions for chinchilla auditory-nerve fibers with characteristic frequencies beyond the phase locking range. Paper presented at 32nd Association for Research in Otolaryngology Midwinter Meeting; February; San Diego, CA. 2009.
-
- Charaziak K, Siegel J. Evaluating frequency selectivity in humans and chinchillas with stimulus-frequency otoacoustic emission tuning curves. Paper presented at 35th Association for Research in Otolaryngology Midwinter Meeting; February; San Diego, CA. 2012.
-
- Cooper NP, Rhode WS. Fast travelling waves, slow travelling waves and their interactions in experimental studies of apical cochlear mechanics. Audit Neurosci. 1996;2:289–299.
-
- Cooper NP, Yates GK. Nonlinear input-output functions derived from the responses of guinea-pig cochlear nerve fibres: variations with characteristic frequency. Hear Res. 1994;78:221–234. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
