Large-scale synchronized activity during vocal deviance detection in the zebra finch auditory forebrain
- PMID: 22855809
- PMCID: PMC6621405
- DOI: 10.1523/JNEUROSCI.6045-11.2012
Large-scale synchronized activity during vocal deviance detection in the zebra finch auditory forebrain
Abstract
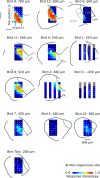
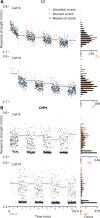
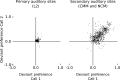
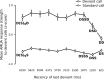
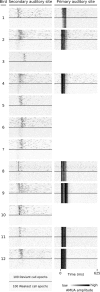
Auditory systems bias responses to sounds that are unexpected on the basis of recent stimulus history, a phenomenon that has been widely studied using sequences of unmodulated tones (mismatch negativity; stimulus-specific adaptation). Such a paradigm, however, does not directly reflect problems that neural systems normally solve for adaptive behavior. We recorded multiunit responses in the caudomedial auditory forebrain of anesthetized zebra finches (Taeniopygia guttata) at 32 sites simultaneously, to contact calls that recur probabilistically at a rate that is used in communication. Neurons in secondary, but not primary, auditory areas respond preferentially to calls when they are unexpected (deviant) compared with the same calls when they are expected (standard). This response bias is predominantly due to sites more often not responding to standard events than to deviant events. When two call stimuli alternate between standard and deviant roles, most sites exhibit a response bias to deviant events of both stimuli. This suggests that biases are not based on a use-dependent decrease in response strength but involve a more complex mechanism that is sensitive to auditory deviance per se. Furthermore, between many secondary sites, responses are tightly synchronized, a phenomenon that is driven by internal neuronal interactions rather than by the timing of stimulus acoustic features. We hypothesize that this deviance-sensitive, internally synchronized network of neurons is involved in the involuntary capturing of attention by unexpected and behaviorally potentially relevant events in natural auditory scenes.
Figures





References
-
- Amin N, Doupe A, Theunissen FE. Development of selectivity for natural sounds in the songbird auditory forebrain. J Neurophysiol. 2007;97:3517–3531. - PubMed
-
- Baayen R, Davidson D, Bates D. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
