Visual fixation as equilibrium: evidence from superior colliculus inactivation
- PMID: 22855812
- PMCID: PMC3473086
- DOI: 10.1523/JNEUROSCI.0696-12.2012
Visual fixation as equilibrium: evidence from superior colliculus inactivation
Abstract
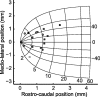
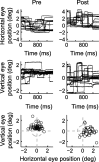
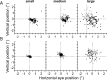
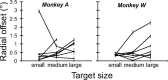
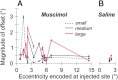
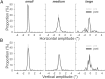
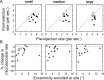
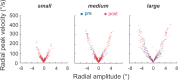
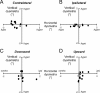
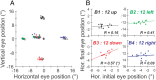
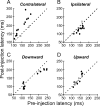
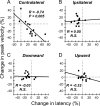
During visual fixation, the image of an object is maintained within the fovea. Previous studies have shown that such maintenance involves the deep superior colliculus (dSC). However, the mechanisms by which the dSC supports visual fixation remain controversial. According to one view, activity in the rostral dSC maintains gaze direction by preventing neurons in the caudal dSC from issuing saccade commands. An alternative hypothesis proposes that gaze direction is achieved through equilibrium of target position signals originating from the two dSCs. Here, we show in monkeys that artificially reducing activity in the rostral half of one dSC results in a biased estimate of target position during fixation, consistent with the second hypothesis, rather than an inability to maintain gaze fixation as predicted by the first hypothesis. After injection of muscimol at rostral sites in the dSC, fixation became more stable since microsaccade rate was reduced rather than increased. Moreover, the scatter of eye positions was offset relative to preinactivation baselines. The magnitude and the direction of the offsets depended on both the target size and the injected site in the collicular map. Other oculomotor parameters, such as the accuracy of saccades to peripheral targets and the amplitude and velocity of fixational saccades, were largely unaffected. These results suggest that the rostral half of the dSC supports visual fixation through a distributed representation of behaviorally relevant target position signals. The inactivation-induced fixation offset establishes the foveal visual stimulation that is required to restore the balance of activity between the two dSCs.
Figures
Similar articles
-
Microsaccade production during saccade cancelation in a stop-signal task.Vision Res. 2016 Jan;118:5-16. doi: 10.1016/j.visres.2014.10.025. Epub 2014 Nov 6. Vision Res. 2016. PMID: 25448116 Free PMC article. Review.
-
Neuronal control of fixation and fixational eye movements.Philos Trans R Soc Lond B Biol Sci. 2017 Apr 19;372(1718):20160205. doi: 10.1098/rstb.2016.0205. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28242738 Free PMC article. Review.
-
Superior colliculus inactivation causes stable offsets in eye position during tracking.J Neurosci. 2008 Aug 6;28(32):8124-37. doi: 10.1523/JNEUROSCI.1317-08.2008. J Neurosci. 2008. PMID: 18685037 Free PMC article.
-
Comparison of saccades perturbed by stimulation of the rostral superior colliculus, the caudal superior colliculus, and the omnipause neuron region.J Neurophysiol. 1999 Dec;82(6):3236-53. doi: 10.1152/jn.1999.82.6.3236. J Neurophysiol. 1999. PMID: 10601457
-
Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus.J Neurophysiol. 1998 Mar;79(3):1193-209. doi: 10.1152/jn.1998.79.3.1193. J Neurophysiol. 1998. PMID: 9497401
Cited by
-
Microsaccade rate as a measure of drug response.J Eye Mov Res. 2019 Oct 3;12(6):10.16910/jemr.12.6.12. doi: 10.16910/jemr.12.6.12. J Eye Mov Res. 2019. PMID: 33828750 Free PMC article.
-
Suppressive interactions underlying visually evoked fixational saccades.Vision Res. 2016 Jan;118:70-82. doi: 10.1016/j.visres.2015.01.009. Epub 2015 Jan 30. Vision Res. 2016. PMID: 25645962 Free PMC article.
-
Microsaccade production during saccade cancelation in a stop-signal task.Vision Res. 2016 Jan;118:5-16. doi: 10.1016/j.visres.2014.10.025. Epub 2014 Nov 6. Vision Res. 2016. PMID: 25448116 Free PMC article. Review.
-
Sequential hemifield gating of α- and β-behavioral performance oscillations after microsaccades.J Neurophysiol. 2017 Nov 1;118(5):2789-2805. doi: 10.1152/jn.00253.2017. Epub 2017 Aug 9. J Neurophysiol. 2017. PMID: 28794193 Free PMC article.
-
Neuronal control of fixation and fixational eye movements.Philos Trans R Soc Lond B Biol Sci. 2017 Apr 19;372(1718):20160205. doi: 10.1098/rstb.2016.0205. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28242738 Free PMC article. Review.
References
-
- Aizawa H, Wurtz RH. Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J Neurophysiol. 1998;79:2082–2096. - PubMed
-
- Albano JE, Wurtz RH. Deficits in eye position following ablation of monkey superior colliculus, pretectum, and posterior-medial thalamus. J Neurophysiol. 1982;48:318–337. - PubMed
-
- Basso MA, Krauzlis RJ, Wurtz RH. Activation and inactivation of rostral superior colliculus neurons during smooth-pursuit eye movements in monkeys. J Neurophysiol. 2000;84:892–908. - PubMed
-
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. - PubMed
-
- Büttner-Ennever JA, Horn AK, Henn V, Cohen B. Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol. 1999;413:55–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
