The stat3/socs3a pathway is a key regulator of hair cell regeneration in zebrafish. [corrected]
- PMID: 22855815
- PMCID: PMC3427933
- DOI: 10.1523/JNEUROSCI.5785-10.2012
The stat3/socs3a pathway is a key regulator of hair cell regeneration in zebrafish. [corrected]
Erratum in
- J Neurosci. 2012 Oct 3;32(40):14052
Abstract
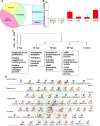
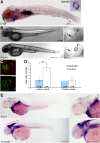
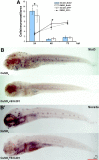
All nonmammalian vertebrates studied can regenerate inner ear mechanosensory receptors (i.e., hair cells) (Corwin and Cotanche, 1988; Lombarte et al., 1993; Baird et al., 1996), but mammals possess only a very limited capacity for regeneration after birth (Roberson and Rubel, 1994). As a result, mammals experience permanent deficiencies in hearing and balance once their inner ear hair cells are lost. The mechanisms of hair cell regeneration are poorly understood. Because the inner ear sensory epithelium is highly conserved in all vertebrates (Fritzsch et al., 2007), we chose to study hair cell regeneration mechanism in adult zebrafish, hoping the results would be transferrable to inducing hair cell regeneration in mammals. We defined the comprehensive network of genes involved in hair cell regeneration in the inner ear of adult zebrafish with the powerful transcriptional profiling technique digital gene expression, which leverages the power of next-generation sequencing ('t Hoen et al., 2008). We also identified a key pathway, stat3/socs3, and demonstrated its role in promoting hair cell regeneration through stem cell activation, cell division, and differentiation. In addition, transient pharmacological inhibition of stat3 signaling accelerated hair cell regeneration without overproducing cells. Taking other published datasets into account (Sano et al., 1999; Schebesta et al., 2006; Dierssen et al., 2008; Riehle et al., 2008; Zhu et al., 2008; Qin et al., 2009), we propose that the stat3/socs3 pathway is a key response in all tissue regeneration and thus an important therapeutic target for a broad application in tissue repair and injury healing.
Figures




Similar articles
-
Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line.J Neurosci. 2008 Feb 27;28(9):2261-73. doi: 10.1523/JNEUROSCI.4372-07.2008. J Neurosci. 2008. PMID: 18305259 Free PMC article.
-
Retinoic Acid Signaling Mediates Hair Cell Regeneration by Repressing p27kip and sox2 in Supporting Cells.J Neurosci. 2015 Nov 25;35(47):15752-66. doi: 10.1523/JNEUROSCI.1099-15.2015. J Neurosci. 2015. PMID: 26609166 Free PMC article.
-
foxg1a is required for hair cell development and regeneration in the zebrafish lateral line.Biol Open. 2024 Sep 15;13(9):bio060580. doi: 10.1242/bio.060580. Epub 2024 Sep 20. Biol Open. 2024. PMID: 39301848 Free PMC article.
-
Sensory hair cell regeneration in the zebrafish lateral line.Dev Dyn. 2014 Oct;243(10):1187-202. doi: 10.1002/dvdy.24167. Epub 2014 Aug 14. Dev Dyn. 2014. PMID: 25045019 Free PMC article. Review.
-
Chemical screening for hair cell loss and protection in the zebrafish lateral line.Zebrafish. 2010 Mar;7(1):3-11. doi: 10.1089/zeb.2009.0639. Zebrafish. 2010. PMID: 20192852 Free PMC article. Review.
Cited by
-
Transcriptome Analyses Reveal IL6/Stat3 Signaling Involvement in Radial Glia Proliferation After Stab Wound Injury in the Adult Zebrafish Optic Tectum.Front Cell Dev Biol. 2021 Apr 30;9:668408. doi: 10.3389/fcell.2021.668408. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996824 Free PMC article.
-
Identification of astroglia-like cardiac nexus glia that are critical regulators of cardiac development and function.PLoS Biol. 2021 Nov 18;19(11):e3001444. doi: 10.1371/journal.pbio.3001444. eCollection 2021 Nov. PLoS Biol. 2021. PMID: 34793438 Free PMC article.
-
Additive reductions in zebrafish PRPS1 activity result in a spectrum of deficiencies modeling several human PRPS1-associated diseases.Sci Rep. 2016 Jul 18;6:29946. doi: 10.1038/srep29946. Sci Rep. 2016. PMID: 27425195 Free PMC article.
-
Copper-induced deregulation of microRNA expression in the zebrafish olfactory system.Environ Sci Technol. 2013 Jul 2;47(13):7466-74. doi: 10.1021/es400615q. Epub 2013 Jun 19. Environ Sci Technol. 2013. PMID: 23745839 Free PMC article.
-
JAK/STAT signaling in stem cells and regeneration: from Drosophila to vertebrates.Development. 2019 Jan 29;146(2):dev167643. doi: 10.1242/dev.167643. Development. 2019. PMID: 30696713 Free PMC article. Review.
References
-
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann NY Acad Sci. 1996;781:59–70. - PubMed
-
- Bang PI, Sewell WF, Malicki JJ. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio) J Comp Neurol. 2001;438:173–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
