Design, synthesis, and preclinical evaluations of novel 4-substituted 1,5-diarylanilines as potent HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) drug candidates
- PMID: 22856541
- PMCID: PMC3462592
- DOI: 10.1021/jm3007678
Design, synthesis, and preclinical evaluations of novel 4-substituted 1,5-diarylanilines as potent HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI) drug candidates
Abstract
Twenty-one new 4-substituted diarylaniline compounds (DAANs) (series 13, 14, and 15) were designed, synthesized, and evaluated against wild-type and drug resistant HIV-1 viral strains. As a result, approximately a dozen new DAANs showed high potency with low nano- to subnanomolar EC(50) values ranging from 0.2 to 10 nM. The three most promising compounds 14e, 14h, and 15h exhibited high potency against wild-type and drug-resistant viral strains with EC(50) values at the subnanomolar level (0.29-0.87 nM) and were comparable to or more potent than the new NNRTI drug riplivirine (2) in the same assays. Druglike physicochemical property assessments revealed that the most active DAANs (EC(50) < 10 nM) have better aqueous solubility (>1-90 μg/mL at pH 7.4 and pH 2) and metabolic stability in vitro than 2, as well as desirable log P values (<5) and polar surface areas (PSA) (<140 Å(2)). These promising results warrant further development of this novel compound class as potential potent anti-AIDS clinical trial candidates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
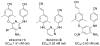

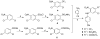

References
-
- Anthony S. Fauci AIDS: let science inform policy. Science. 2011;333(6038):13. - PubMed
-
- Shattock RJ, Warren M, McCormack S, Hankins C. Turning the tide against HIV. Science. 2011;333(6038):42–43. - PubMed
-
- Kaufmann GR, Cooper DA. Antiretroviral therapy of HIV-1 infection: established treatment strategies and new therapeutic options. Curr Opin Microbiol. 2000;3:508–514. - PubMed
-
- Vella S, Palmisano L. Antiretroviral therapy: state of the HAART. Antiviral Res. 2000;45:1–7. - PubMed
-
- Pecora Fulco P, McNicholl IR. Etravirine and rilpivirine: nonnucleoside reverse transcriptase inhibitors with activity against human immunodeficiency virus type 1 strains resistant to previous nonnucleoside agents. Pharmacotherapy. 2009;29 (3):281–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

