Mechanisms of glioma-associated neovascularization
- PMID: 22858156
- PMCID: PMC3463636
- DOI: 10.1016/j.ajpath.2012.06.030
Mechanisms of glioma-associated neovascularization
Abstract
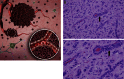
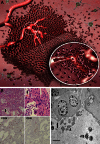
Glioblastomas (GBMs), the most common primary brain tumor in adults, are characterized by resistance to chemotherapy and radiotherapy. One of the defining characteristics of GBM is an abundant and aberrant vasculature. The processes of vascular co-option, angiogenesis, and vasculogenesis in gliomas have been extensively described. Recently, however, it has become clear that these three processes are not the only mechanisms by which neovascularization occurs in gliomas. Furthermore, it seems that these processes interact extensively, with potential overlap among them. At least five mechanisms by which gliomas achieve neovascularization have been described: vascular co-option, angiogenesis, vasculogenesis, vascular mimicry, and (the most recently described) glioblastoma-endothelial cell transdifferentiation. We review these mechanisms in glioma neovascularization, with a particular emphasis on the roles of hypoxia and glioma stem cells in each process. Although some of these processes are well established, others have been identified only recently and will need to be further investigated for complete validation. We also review strategies to target glioma neovascularization and the development of resistance to these therapeutic strategies. Finally, we describe how these complex processes interlink and overlap. A thorough understanding of the contributing molecular processes that control the five modalities reviewed here should help resolve the treatment resistance that characterizes GBMs.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Brem S., Cotran R., Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. - PubMed
-
- Holash J., Maisonpierre P.C., Compton D., Boland P., Alexander C.R., Zagzag D., Yancopoulos G.D., Wiegand S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

