Dorsal root ganglion neurons innervating pelvic organs in the mouse express tyrosine hydroxylase
- PMID: 22858598
- PMCID: PMC3491663
- DOI: 10.1016/j.neuroscience.2012.07.043
Dorsal root ganglion neurons innervating pelvic organs in the mouse express tyrosine hydroxylase
Abstract
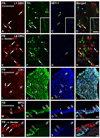
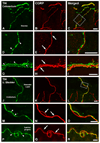
Previous studies in rat and mouse documented that a subpopulation of dorsal root ganglion (DRG) neurons innervating non-visceral tissues express tyrosine hydroxylase (TH). Here we studied whether or not mouse DRG neurons retrogradely traced with Fast Blue (FB) from colorectum or urinary bladder also express immunohistochemically detectable TH. The lumbar sympathetic chain (LSC) and major pelvic ganglion (MPG) were included in the analysis. Previously characterized antibodies against TH, norepinephrine transporter type 1 (NET-1) and calcitonin gene-related peptide (CGRP) were used. On average, ∼14% of colorectal and ∼17% of urinary bladder DRG neurons expressed TH and spanned virtually all neuronal sizes, although more often in the medium-sized to small ranges. Also, they were more abundant in lumbosacral than thoracolumbar DRGs, and often coexpressed CGRP. We also detected several TH-immunoreactive (IR) colorectal and urinary bladder neurons in the LSC and the MPG, more frequently in the former. No NET-1-IR neurons were detected in DRGs, whereas the majority of FB-labeled, TH-IR neurons in the LSC and MPG coexpressed this marker (as did most other TH-IR neurons not labeled from the target organs). TH-IR nerve fibers were detected in all layers of the colorectum and the urinary bladder, with some also reaching the basal mucosal cells. Most TH-IR fibers in these organs lacked CGRP. Taken together, we show: (1) that a previously undescribed population of colorectal and urinary bladder DRG neurons expresses TH, often CGRP but not NET-1, suggesting the absence of a noradrenergic phenotype; and (2) that TH-IR axons/terminals in the colon or urinary bladder, naturally expected to derive from autonomic sources, could also originate from sensory neurons.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
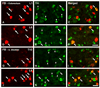

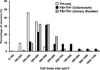
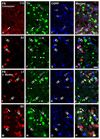

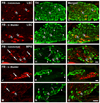

Similar articles
-
Dorsal root ganglion neurons and tyrosine hydroxylase--an intriguing association with implications for sensation and pain.Pain. 2016 Feb;157(2):314-320. doi: 10.1097/j.pain.0000000000000381. Pain. 2016. PMID: 26447702 Free PMC article. Review.
-
Expression of vesicular glutamate transporters type 1 and 2 in sensory and autonomic neurons innervating the mouse colorectum.J Comp Neurol. 2011 Nov 1;519(16):3346-66. doi: 10.1002/cne.22730. J Comp Neurol. 2011. PMID: 21800314 Free PMC article.
-
Distribution and neurochemical characterization of sensory dorsal root ganglia neurons supplying porcine urinary bladder.J Physiol Pharmacol. 2009 Oct;60 Suppl 4:77-81. J Physiol Pharmacol. 2009. PMID: 20083855
-
Tyrosine hydroxylase is expressed in a subpopulation of small dorsal root ganglion neurons in the adult mouse.Exp Neurol. 2006 Jul;200(1):153-65. doi: 10.1016/j.expneurol.2006.01.023. Epub 2006 Mar 6. Exp Neurol. 2006. PMID: 16516890
-
Distribution and immunocytochemical characterization of dorsal root ganglion neurons innervating the lumbar intervertebral disc in rats: a review.Life Sci. 2004 Apr 9;74(21):2627-42. doi: 10.1016/j.lfs.2004.01.008. Life Sci. 2004. PMID: 15041445 Review.
Cited by
-
Dorsal root ganglion neurons and tyrosine hydroxylase--an intriguing association with implications for sensation and pain.Pain. 2016 Feb;157(2):314-320. doi: 10.1097/j.pain.0000000000000381. Pain. 2016. PMID: 26447702 Free PMC article. Review.
-
Dynamic Expression of Serotonin Receptor 5-HT3A in Developing Sensory Innervation of the Lower Urinary Tract.Front Neurosci. 2017 Jan 6;10:592. doi: 10.3389/fnins.2016.00592. eCollection 2016. Front Neurosci. 2017. PMID: 28111539 Free PMC article.
-
Comprehensive mapping of sensory and sympathetic innervation of the developing kidney.Cell Rep. 2024 Oct 22;43(10):114860. doi: 10.1016/j.celrep.2024.114860. Epub 2024 Oct 15. Cell Rep. 2024. PMID: 39412983 Free PMC article.
-
Nociceptive and inflammatory mediator upregulation in a mouse model of chronic prostatitis.Pain. 2015 Aug;156(8):1537-1544. doi: 10.1097/j.pain.0000000000000201. Pain. 2015. PMID: 25915147 Free PMC article.
-
Sympathetic axonopathies and hyperinnervation in the small intestine smooth muscle of aged Fischer 344 rats.Auton Neurosci. 2013 Dec;179(1-2):108-21. doi: 10.1016/j.autneu.2013.09.002. Epub 2013 Sep 18. Auton Neurosci. 2013. PMID: 24104187 Free PMC article.
References
-
- Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–1463. - PubMed
-
- Biallosterski BT, de Wachter SG, van Koeveringe GA, van Kerrebroeck PE, DE VJ, Mulder MT, Gillespie JI. Changes in bladder innervation in a mouse model of Alzheimer's disease. J. Chem. Neuroanat. 2010;39:204–210. - PubMed
-
- Brierley SM, Jones RC, III, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178. - PubMed
-
- Brookes SJ, Dinning PG, Gladman MA. Neuroanatomy and physiology of colorectal function and defaecation: from basic science to human clinical studies. Neurogastroenterol. Motil. 2009;21(Suppl 2):9–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

