A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial
- PMID: 22859796
- PMCID: PMC3895957
- DOI: 10.1093/eurheartj/ehs232
A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial
Abstract
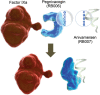
Aims: We sought to determine the degree of anticoagulation reversal required to mitigate bleeding, and assess the feasibility of using pegnivacogin to prevent ischaemic events in acute coronary syndrome (ACS) patients managed with an early invasive approach. REG1 consists of pegnivacogin, an RNA aptamer selective factor IXa inhibitor, and its complementary controlling agent, anivamersen. REG1 has not been studied in invasively managed patients with ACS nor has an optimal level of reversal allowing safe sheath removal been defined.
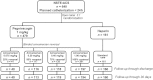
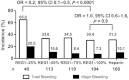
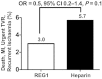
Methods and results: Non-ST-elevation ACS patients (n = 640) with planned early cardiac catheterization via femoral access were randomized 2:1:1:2:2 to pegnivacogin with 25, 50, 75, or 100% anivamersen reversal or heparin. The primary endpoint was total ACUITY bleeding through 30 days. Secondary endpoints included major bleeding and the composite of death, myocardial infarction, urgent target vessel revascularization, or recurrent ischaemia. Enrolment in the 25% reversal arm was suspended after 41 patients. Enrolment was stopped after three patients experienced allergic-like reactions. Bleeding occurred in 65, 34, 35, 30, and 31% of REG1 patients with 25, 50, 75, and 100% reversal and heparin. Major bleeding occurred in 20, 11, 8, 7, and 10% of patients. Ischaemic events occurred in 3.0 and 5.7% of REG1 and heparin patients, respectively.
Conclusion: At least 50% reversal is required to allow safe sheath removal after cardiac catheterization. REG1 appears a safe strategy to anticoagulate ACS patients managed invasively and warrants further investigation in adequately powered clinical trials of patients who require short-term high-intensity anticoagulation.
Keywords: Acute coronary syndromes; Anticoagulation reversal; REG1.
Figures

References
-
- Becker RC, Rusconi C, Sullenger B. Nucleic acid aptamers in therapeutic anticoagulation. Technology, development, and clinical application. Thromb Haemost. 2005;93:1014–1020. - PubMed
-
- Becker RC, Povsic TJ, Cohen MG, Rusconi CP, Sullenger BA. Nucleic acid aptamers as antithrombotic agents: opportunities in extracellular therapeutics. Thromb Haemost. 2010;103:586–595. doi:10.1160/TH09-10-0716. - DOI - PubMed
-
- Povsic T, Sullenger B, Zelenkofske S, Rusconi C, Becker R. Translating nucleic acid aptamers to antithrombotic drugs in cardiovascular medicine. J Cardiovasc Transl Res. 2011;3:704–716. doi:10.1007/s12265-010-9230-6. - DOI - PubMed
-
- Povsic TJ, Cohen MG, Chan MY, Zelenkofske SL, Wargin WA, Harrington RA, Alexander JH, Rusconi CP, Becker RC. Dose selection for a direct and selective factor IXa inhibitor and its complementary reversal agent: translating pharmacokinetic and pharmacodynamic properties of the REG1 system to clinical trial design. J Thromb Thrombolysis. 2011;32:21–31. doi:10.1007/s11239-011-0588-3. - DOI - PubMed
-
- Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, Alexander JH, Becker RC, Rusconi CP. First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 2006;114:2490–2497. doi:10.1161/CIRCULATIONAHA.106.668434. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

