Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons
- PMID: 22859819
- PMCID: PMC3490628
- DOI: 10.1126/science.1222826
Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons
Abstract
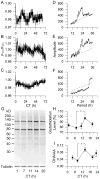
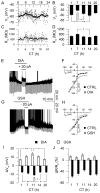
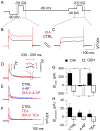
Daily rhythms of mammalian physiology, metabolism, and behavior parallel the day-night cycle. They are orchestrated by a central circadian clock in the brain, the suprachiasmatic nucleus (SCN). Transcription of clock genes is sensitive to metabolic changes in reduction and oxidation (redox); however, circadian cycles in protein oxidation have been reported in anucleate cells, where no transcription occurs. We investigated whether the SCN also expresses redox cycles and how such metabolic oscillations might affect neuronal physiology. We detected self-sustained circadian rhythms of SCN redox state that required the molecular clockwork. The redox oscillation could determine the excitability of SCN neurons through nontranscriptional modulation of multiple potassium (K(+)) channels. Thus, dynamic regulation of SCN excitability appears to be closely tied to metabolism that engages the clockwork machinery.
Figures

Comment in
-
Physiology. Circadian time redoxed.Science. 2012 Aug 17;337(6096):805-6. doi: 10.1126/science.1227203. Science. 2012. PMID: 22904000 No abstract available.
References
-
- Prosser RA, Gillette MU. Cyclic changes in cAMP concentration and phosphodiesterase activity in a mammalian circadian clock studied in vitro. Brain Res. 1991;568:185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

