Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency
- PMID: 22859821
- PMCID: PMC3507439
- DOI: 10.1126/science.1224026
Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency
Abstract
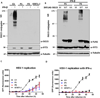
ISG15 is an interferon (IFN)-α/β-inducible, ubiquitin-like intracellular protein. Its conjugation to various proteins (ISGylation) contributes to antiviral immunity in mice. Here, we describe human patients with inherited ISG15 deficiency and mycobacterial, but not viral, diseases. The lack of intracellular ISG15 production and protein ISGylation was not associated with cellular susceptibility to any viruses that we tested, consistent with the lack of viral diseases in these patients. By contrast, the lack of mycobacterium-induced ISG15 secretion by leukocytes-granulocyte, in particular-reduced the production of IFN-γ by lymphocytes, including natural killer cells, probably accounting for the enhanced susceptibility to mycobacterial disease. This experiment of nature shows that human ISGylation is largely redundant for antiviral immunity, but that ISG15 plays an essential role as an IFN-γ-inducing secreted molecule for optimal antimycobacterial immunity.
Figures


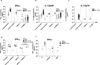
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

