Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury
- PMID: 22859854
- PMCID: PMC3431420
- DOI: 10.1681/ASN.2012020137
Activation of parenchymal CD47 promotes renal ischemia-reperfusion injury
Abstract
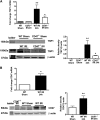
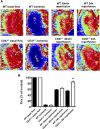
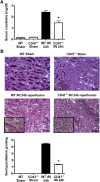
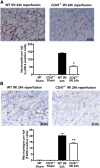
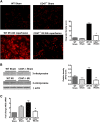
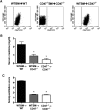
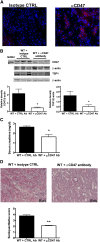
Ischemia-reperfusion injury (IRI) contributes to decreased allograft function and allograft rejection in transplanted kidneys. Thrombospondin-1 is a stress protein typically secreted in response to hypoxia and the ligand activator for the ubiquitously expressed receptor CD47. The function of activated CD47 in IRI remains completely unknown. Here, we found that both CD47 and its ligand thrombospondin-1 were upregulated after renal IRI in mice. CD47-knockout mice were protected against renal dysfunction and tubular damage, suggesting that the development of IRI requires intact CD47 signaling. Chimeric CD47-knockout mice engrafted with wild-type hematopoietic cells had significantly lower serum creatinine and less tubular damage than wild-type controls after IRI, suggesting that CD47 signaling in parenchymal cells predominantly mediates renal damage. Treatment with a CD47-blocking antibody protected mice from renal dysfunction and tubular damage compared with an isotype control. Taken together, these data imply that CD47 on parenchymal cells promotes injury after renal ischemia and reperfusion. Therefore, CD47 blockade may have therapeutic potential to prevent or suppress ischemia-reperfusion-mediated damage.
Figures





References
-
- Halloran PF, Homik J, Goes N, Lui SL, Urmson J, Ramassar V, Cockfield SM: The “injury response”: A concept linking nonspecific injury, acute rejection, and long-term transplant outcomes. Transplant Proc 29: 79–81, 1997 - PubMed
-
- Hetzel GR, Klein B, Brause M, Westhoff A, Willers R, Sandmann W, Grabensee B: Risk factors for delayed graft function after renal transplantation and their significance for long-term clinical outcome. Transpl Int 15: 10–16, 2002 - PubMed
-
- Shoskes DA, Halloran PF: Delayed graft function in renal transplantation: Etiology, management and long-term significance. J Urol 155: 1831–1840, 1996 - PubMed
-
- Smadja DM, d’Audigier C, Bièche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P: Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol 31: 551–559, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

