Neuronal transcription factors induce conversion of human glioma cells to neurons and inhibit tumorigenesis
- PMID: 22859994
- PMCID: PMC3409237
- DOI: 10.1371/journal.pone.0041506
Neuronal transcription factors induce conversion of human glioma cells to neurons and inhibit tumorigenesis
Abstract
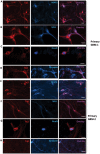
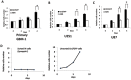
Recent findings have demonstrated that the overexpression of lineage-specific transcription factors induces cell fate changes among diverse cell types. For example, neurons can be generated from mouse and human fibroblasts. It is well known that neurons are terminally differentiated cells that do not divide. Therefore, we consider how to induce glioma cells to become neurons by introducing transcription factors. Here, we describe the efficient generation of induced neuronal (iN) cells from glioma cells by the infection with three transcription factors: Ascl1, Brn2 and Ngn2 (ABN). iN cells expressed multiple neuronal markers and fired action potentials, similar to the properties of authentic neurons. Importantly, the proliferation of glioma cells following ABN overexpression was dramatically inhibited in both in vitro and in vivo experiments. In addition, iN cells that originated from human glioma cells did not continue to grow when they were sorted and cultured in vitro. The strategies by which glioma cells are induced to become neurons may be used to clinically study methods for inhibiting tumor growth.
Conflict of interest statement
Figures




References
-
- Dell’Albani P (2008) Stem cell markers in gliomas. Neurochem Res 33: 2407–2415. - PubMed
-
- Sukhdeo K, Hambardzumyan D, Rich JN (2011) Glioma development: where did it all go wrong? Cell 146: 187–188. - PubMed
-
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361: 323–331. - PubMed
-
- Norden AD, Drappatz J, Wen PY (2009) Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol 5: 610–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

