Antibodies to the α1-adrenergic receptor cause vascular impairments in rat brain as demonstrated by magnetic resonance angiography
- PMID: 22860001
- PMCID: PMC3408502
- DOI: 10.1371/journal.pone.0041602
Antibodies to the α1-adrenergic receptor cause vascular impairments in rat brain as demonstrated by magnetic resonance angiography
Abstract
Background: Circulating agonistic autoantibodies acting at G protein-coupled receptors have been associated with numerous sever pathologies in humans. Antibodies directed predominantly against the α(1)-adrenergig receptor were detected in patients suffering from widespread diseases such as hypertension and type 2 diabetes. Their deleterious action has been demonstrated for peripheral organs. We postulate that antibodies to the α(1)-adrenergig receptor are relevant pathomolecules in diseases of the central nervous system associated with vascular impairments.
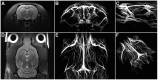
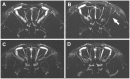
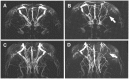
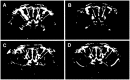
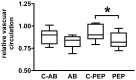
Methodology/principal findings: Using a rat model we studied the long-term action of antibodies against the α(1)-adrenergig receptor either induced by immunization with a receptor peptide or applied by intravenous injection. The vasculature in the rat brains was investigated by time-of-flight magnetic resonance angiography using a 9.4 Tesla small animal MR imaging system. Visual examination of maximum-intensity-projections (MIPs) of brain angiographs revealed the development of vascular defects in antibody- exposed animals between three and eight months of treatment. Relative vascular areas were derived from representative MIP image sections by grayscale analysis and used to form an index of vascular circulation. Animals exposed to the action of α(1)-adrenergig receptor antibodies showed significantly reduced vascular areas (p<0.05). Calculated index values indicated attenuated blood flow in both antibody-treated cohorts compared to their respective controls reaching with (relative units ± standard error, n = 10) 0.839 ± 0.026 versus 0.919 ± 0.026 statistical significance (p<0.05) for peptide-immunized rats.
Conclusion/significance: We present evidence that antibodies to the α(1)-adrenergig receptor cause cerebrovascular impairments in the rat. Our findings suggest the pathological significance of these antibodies in pathologies of the human central nervous system linked to impairments of brain vasculature such as stroke and dementia.
Conflict of interest statement
Figures

References
-
- De la Torre (2002) Alzheimer disease as a vascular disorder: nosological evidence. Stroke 33: 1152–1162. - PubMed
-
- Dragun D, Philippe A, Catar R, Hegner B (2009) Autoimmune mediated G-protein receptor activation in cardiovascular and renal pathologies. Thromb Haemost 101: 643–648. - PubMed
-
- Kaya Z, Lein C, Katus H (2012) Autoantibodies in heart failure and cardiac dysfunction. Circulation Research 110: 145–158. - PubMed
-
- Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J (1994) Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 89: 2760–2767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

