Long-term hepatitis B virus (HBV) response to lamivudine-containing highly active antiretroviral therapy in HIV-HBV co-infected patients in Thailand
- PMID: 22860080
- PMCID: PMC3409123
- DOI: 10.1371/journal.pone.0042184
Long-term hepatitis B virus (HBV) response to lamivudine-containing highly active antiretroviral therapy in HIV-HBV co-infected patients in Thailand
Abstract
Background: Approximately 4 million of people are co-infected with HIV and Hepatitis B virus (HBV). In resource-limited settings, the majority of HIV-infected patients initiate first-line highly active antiretroviral therapy containing lamivudine (3TC-containing-HAART) and long-term virological response of HBV to lamivudine-containing HAART in co-infected patients is not well known.
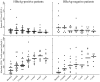
Methodology/principal finding: HIV-HBV co-infected patients enrolled in the PHPT cohort (ClinicalTrials.gov NCT00433030) and initiating a 3TC-containing-HAART regimen were included. HBV-DNA, HIV-RNA, CD4+ T-cell counts and alanine transaminase were measured at baseline, 3 months, 12 months and then every 6 months up to 5 years. Kaplan-Meier analysis was used to estimate the cumulative rates of patients who achieved and maintained HBV-DNA suppression. Of 30 co-infected patients, 19 were positive for HBe antigen (HBeAg). At initiation of 3TC-containing-HAART, median HBV DNA and HIV RNA levels were 7.35 log(10) IU/mL and 4.47 log(10) copies/mL, respectively. At 12 months, 67% of patients achieved HBV DNA suppression: 100% of HBeAg-negative patients and 47% of HBeAg-positive. Seventy-three percent of patients had HIV RNA below 50 copies/mL. The cumulative rates of maintained HBV-DNA suppression among the 23 patients who achieved HBV-DNA suppression were 91%, 87%, and 80% at 1, 2, and 4 years respectively. Of 17 patients who maintained HBV-DNA suppression while still on 3TC, 4 (24%) lost HBsAg and 7 of 8 (88%) HBeAg-positive patients lost HBeAg at their last visit (median duration, 59 months). HBV breakthrough was observed only in HBeAg-positive patients and 6 of 7 patients presenting HBV breakthrough had the rtM204I/V mutations associated with 3TC resistance along with rtL180M and/or rtV173L.
Conclusions: All HBeAg-negative patients and 63% of HBeAg-positive HIV-HBV co-infected patients achieved long-term HBV DNA suppression while on 3TC-containing-HAART. This study provides information useful for the management of co-infected patients in resource-limited countries where the vast majority of co-infected patients are currently receiving 3TC.
Conflict of interest statement
Figures


References
-
- World Health Organization (WHO) (2011) Global HIV/AIDS Response - Epidemic update and health sector progress towards universal access - Progress report 2011. 12 p.
-
- Alter MJ (2006) Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 44: S6–9. - PubMed
-
- Thio CL, Seaberg EC, Skolasky R Jr, Phair J, Visscher B, et al. (2002) HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 360: 1921–1926. - PubMed
-
- Nikolopoulos GK, Paraskevis D, Hatzitheodorou E, Moschidis Z, Sypsa V, et al. (2009) Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: a cohort study and meta-analysis. Clin Infect Dis 48: 1763–1771. - PubMed
-
- Sungkanuparph S, Techasathit W, Utaipiboon C, Chasombat S, Bhakeecheep S, et al. (2010) Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomedicine 4: 515–528.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

