Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis
- PMID: 22860081
- PMCID: PMC3408460
- DOI: 10.1371/journal.pone.0042199
Subcellular localization of iron and heme metabolism related proteins at early stages of erythrophagocytosis
Abstract
Background: Senescent red blood cells (RBC) are recognized, phagocytosed and cleared by tissue macrophages. During this erythrophagocytosis (EP), RBC are engulfed and processed in special compartments called erythrophagosomes. We previously described that following EP, heme is rapidly degraded through the catabolic activity of heme oxygenase (HO). Extracted heme iron is then either exported or stored by macrophages. However, the cellular localization of the early steps of heme processing and iron extraction during EP remains to be clearly defined.
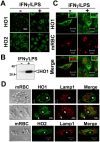
Methodology/principal findings: We took advantage of our previously described cellular model of EP, using bone marrow-derived macrophages (BMDM). The subcellular localization of both inducible and constitutive isoforms of HO (HO-1 and HO-2), of the divalent metal transporters (Nramp1, Nramp2/DMT1, Fpn), and of the recently identified heme transporter HRG-1, was followed by fluorescence and electron microscopy during the earliest steps of EP. We also looked at some ER [calnexin, glucose-6-phosphatase (G6Pase) activity] and lysosomes (Lamp1) markers during EP. In both quiescent and LPS-activated BMDM, Nramp1 and Lamp1 were shown to be strong markers of the erythrophagolysosomal membrane. HRG-1 was also recruited to the erythrophagosome. Furthermore, we observed calnexin labeling and G6Pase activity at the erythrophagosomal membrane, indicating the contribution of ER in this phagocytosis model. In contrast, Nramp2/DMT1, Fpn, HO-1 and HO-2 were not detected at the membrane of erythrophagosomes.
Conclusions/significance: Our study highlights the subcellular localization of various heme- and iron-related proteins during early steps of EP, thereby suggesting a model for heme catabolism occurring outside the phagosome, with heme likely being transported into the cytosol through HRG1. The precise function of Nramp1 at the phagosomal membrane in this model remains to be determined.
Conflict of interest statement
Figures





References
-
- Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, et al. (1998) Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80: 173–195. - PubMed
-
- Deiss A (1999) editor (1999) Destruction of erythrocytes. Lipincott Williams & Wilkins, Baltimore, MD. ed: Lee GR, Foerster J, Lukens J, Paraskevas F,Greer JP, and Rodgers, GM. 267–299 p.
-
- Knutson M, Wessling-Resnick M (2003) Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol 38: 61–88. - PubMed
-
- Beaumont C, Canonne-Hergaux F (2005) [Erythrophagocytosis and recycling of heme iron in normal and pathological conditions; regulation by hepcidin]. Transfus Clin Biol 12: 123–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

