Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin
- PMID: 22860104
- PMCID: PMC3409181
- DOI: 10.1371/journal.pone.0042276
Effects of niacin restriction on sirtuin and PARP responses to photodamage in human skin
Abstract
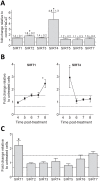
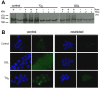
Sirtuins (SIRTs) and poly(ADP-ribose) polymerases (PARPs), NAD(+)-dependent enzymes, link cellular energy status with responses to environmental stresses. Skin is frequently exposed to the DNA damaging effects of UV irradiation, a known etiology in skin cancer. Thus, understanding the defense mechanisms in response to UV, including the role of SIRTs and PARPs, may be important in developing skin cancer prevention strategies. Here, we report expression of the seven SIRT family members in human skin. SIRTs gene expressions are progressively upregulated in A431 epidermoid carcinoma cells (SIRTs1 and 3), actinic keratoses (SIRTs 2, 3, 5, 6, and 7) and squamous cell carcinoma (SIRTs 1-7). Photodamage induces dynamic changes in SIRT expression with upregulation of both SIRT1 and SIRT4 mRNAs. Specific losses of SIRT proteins occur early after photodamage followed by accumulation later, especially for SIRT4. Niacin restriction, which decreases NAD(+), the sirtuin substrate, results in an increase in acetylated proteins, upregulation of SIRTs 2 and 4, increased inherent DNA damage, alterations in SIRT responses to photodamage, abrogation of PARP activation following photodamage, and increased sensitivity to photodamage that is completely reversed by repleting niacin. These data support the hypothesis that SIRTs and PARPs play important roles in resistance to photodamage and identify specific SIRTs that respond to photodamage and may be targets for skin cancer prevention.
Conflict of interest statement
Figures




References
-
- Spronck JC, Kirkland JB (2002) Niacin deficiency increases spontaneous and etoposide-induced chromosomal instability in rat bone marrow cells in vivo. Mutat Res 508: 83–97. - PubMed
-
- Zhang JZ, Henning SM, Swendseid ME (1993) Poly(ADP-ribose) polymerase activity and DNA strand breaks are affected in tissues of niacin-deficient rats. J Nutr 123: 1349–1355. - PubMed
-
- Spronck JC, Nickerson JL, Kirkland JB (2007) Niacin Deficiency Alters p53 Expression and Impairs Etoposide-Induced Cell Cycle Arrest and Apoptosis in Rat Bone Marrow Cells. Nutr Cancer 57: 88–99. - PubMed
-
- Spronck JC, Bartleman AP, Boyonoski AC, Kirkland JB (2003) Chronic DNA damage and niacin deficiency enhance cell injury and cause unusual interactions in NAD and poly(ADP-ribose) metabolism in rat bone marrow. Nutr Cancer 45: 124–131. - PubMed
-
- Boyonoski AC, Gallacher LM, ApSimon MM, Jacobs RM, Shah GM, et al. (1999) Niacin deficiency increases the sensitivity of rats to the short and long term effects of ethylnitrosourea treatment. Mol Cell Biochem 193: 83–87. - PubMed

