Yeasts acquire resistance secondary to antifungal drug treatment by adaptive mutagenesis
- PMID: 22860105
- PMCID: PMC3409178
- DOI: 10.1371/journal.pone.0042279
Yeasts acquire resistance secondary to antifungal drug treatment by adaptive mutagenesis
Abstract
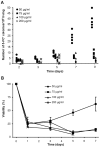
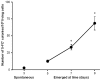
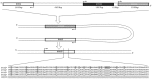
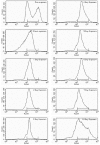
Acquisition of resistance secondary to treatment both by microorganisms and by tumor cells is a major public health concern. Several species of bacteria acquire resistance to various antibiotics through stress-induced responses that have an adaptive mutagenesis effect. So far, adaptive mutagenesis in yeast has only been described when the stress is nutrient deprivation. Here, we hypothesized that adaptive mutagenesis in yeast (Saccharomyces cerevisiae and Candida albicans as model organisms) would also take place in response to antifungal agents (5-fluorocytosine or flucytosine, 5-FC, and caspofungin, CSP), giving rise to resistance secondary to treatment with these agents. We have developed a clinically relevant model where both yeasts acquire resistance when exposed to these agents. Stressful lifestyle associated mutation (SLAM) experiments show that the adaptive mutation frequencies are 20 (S. cerevisiae -5-FC), 600 (C. albicans -5-FC) or 1000 (S. cerevisiae--CSP) fold higher than the spontaneous mutation frequency, the experimental data for C. albicans -5-FC being in agreement with the clinical data of acquisition of resistance secondary to treatment. The spectrum of mutations in the S. cerevisiae -5-FC model differs between spontaneous and acquired, indicating that the molecular mechanisms that generate them are different. Remarkably, in the acquired mutations, an ectopic intrachromosomal recombination with an 87% homologous gene takes place with a high frequency. In conclusion, we present here a clinically relevant adaptive mutation model that fulfils the conditions reported previously.
Conflict of interest statement
Figures


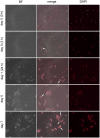
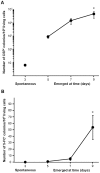
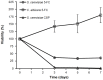
References
-
- Lewin CS, Amyes SG (1991) The role of the SOS response in bacteria exposed to zidovudine or trimethoprim. J Med Microbiol 34: 329–332. - PubMed
-
- Miller C, Thomsen LE, Gaggero C, Mosseri R, Ingmer H, et al. (2004) SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science 305: 1629–1631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

