Vaccination with embryonic stem cells protects against lung cancer: is a broad-spectrum prophylactic vaccine against cancer possible?
- PMID: 22860107
- PMCID: PMC3409174
- DOI: 10.1371/journal.pone.0042289
Vaccination with embryonic stem cells protects against lung cancer: is a broad-spectrum prophylactic vaccine against cancer possible?
Abstract
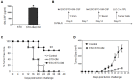
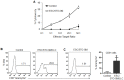
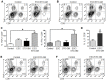
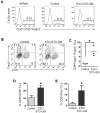
The antigenic similarity between tumors and embryos has been appreciated for many years and reflects the expression of embryonic gene products by cancer cells and/or cancer-initiating stem cells. Taking advantage of this similarity, we have tested a prophylactic lung cancer vaccine composed of allogeneic murine embryonic stem cells (ESC). Naïve C57BL/6 mice were vaccinated with ESC along with a source of granulocyte macrophage-colony stimulating factor (GM-CSF) in order to provide immunostimulatory adjuvant activity. Vaccinated mice were protected against subsequent challenge with implantable Lewis lung carcinoma (LLC). ESC-induced anti-tumor immunity was not due to a non-specific "allo-response" as vaccination with allogeneic murine embryonic fibroblasts did not protect against tumor outgrowth. Vaccine efficacy was associated with robust tumor-reactive primary and memory CD8(+) T effector responses, Th1 cytokine response, higher intratumoral CD8(+) T effector/CD4(+)CD25(+)Foxp3(+) T regulatory cell ratio, and reduced myeloid derived suppressor cells in the spleen. Prevention of tumorigenesis was found to require a CD8-mediated cytotoxic T lymphocyte (CTL) response because in vivo depletion of CD8(+) T lymphocytes completely abrogated the protective effect of vaccination. Importantly, this vaccination strategy also suppressed the development of lung cancer induced by the combination of carcinogen administration and chronic pulmonary inflammation. Further refinement of this novel vaccine strategy and identification of shared ESC/tumor antigens may lead to immunotherapeutic options for lung cancer patients and, perhaps more importantly, could represent a first step toward the development of prophylactic cancer vaccines.
Conflict of interest statement
Figures


References
-
- Triolo VA (1965) Nineteenth Century Foundations of Cancer Research Advances in Tumor Pathology, Nomenclature, and Theories of Oncogenesis. Cancer Res 25: 75–106. - PubMed
-
- Al-Hajj M, Clarke MF (2004) Self-renewal and solid tumor stem cells. Oncogene 23: 7274–7282. - PubMed
-
- Stonehill EH, Bendich A (1970) Retrogenetic expression: the reappearance of embryonal antigens in cancer cells. Nature 228: 370–372. - PubMed
-
- Baldwin RW, Glaves D, Vose BM (1972) Embryonic antigen expression in chemically induced rat hepatomas and sarcomas. Int J Cancer 10: 233–243. - PubMed
-
- Baldwin RW, Glaves D, Pimm MV, Vose BM (1972) Tumour specific and embryonic antigen expression of chemically induced rat tumours. Ann Inst Pasteur (Paris) 122: 715–728. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

