Identification of rat ventral tegmental area GABAergic neurons
- PMID: 22860119
- PMCID: PMC3409171
- DOI: 10.1371/journal.pone.0042365
Identification of rat ventral tegmental area GABAergic neurons
Abstract
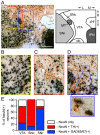
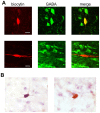
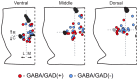
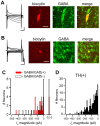
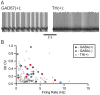
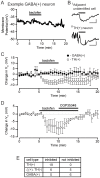
The canonical two neuron model of opioid reward posits that mu opioid receptor (MOR) activation produces reward by disinhibiting midbrain ventral tegmental area (VTA) dopamine neurons through inhibition of local GABAergic interneurons. Although indirect evidence supports the neural circuit postulated by this model, its validity has been called into question by growing evidence for VTA neuronal heterogeneity and the recent demonstration that MOR agonists inhibit GABAergic terminals in the VTA arising from extrinsic neurons. In addition, VTA MOR reward can be dopamine-independent. To directly test the assumption that MOR activation directly inhibits local GABAergic neurons, we investigated the properties of rat VTA GABA neurons directly identified with either immunocytochemistry for GABA or GAD65/67, or in situ hybridization for GAD65/67 mRNA. Utilizing co-labeling with an antibody for the neural marker NeuN and in situ hybridization against GAD65/67, we found that 23±3% of VTA neurons are GAD65/67(+). In contrast to the assumptions of the two neuron model, VTA GABAergic neurons are heterogeneous, both physiologically and pharmacologically. Importantly, only 7/13 confirmed VTA GABA neurons were inhibited by the MOR selective agonist DAMGO. Interestingly, all confirmed VTA GABA neurons were insensitive to the GABA(B) receptor agonist baclofen (0/6 inhibited), while all confirmed dopamine neurons were inhibited (19/19). The heterogeneity of opioid responses we found in VTA GABAergic neurons, and the fact that GABA terminals arising from neurons outside the VTA are inhibited by MOR agonists, make further studies essential to determine the local circuit mechanisms underlying VTA MOR reward.
Conflict of interest statement
Figures




Similar articles
-
GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis.Eur J Neurosci. 2014 Jun;39(11):1796-809. doi: 10.1111/ejn.12503. Epub 2014 Mar 2. Eur J Neurosci. 2014. PMID: 24580812
-
Presynaptic mu-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons.Neuropharmacology. 2002 Jun;42(8):1065-78. doi: 10.1016/s0028-3908(02)00061-8. Neuropharmacology. 2002. PMID: 12128008
-
GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems.Eur J Neurosci. 2001 Mar;13(5):1009-15. doi: 10.1046/j.1460-9568.2001.01458.x. Eur J Neurosci. 2001. PMID: 11264674
-
Functional diversity of ventral midbrain dopamine and GABAergic neurons.Mol Neurobiol. 2004 Jun;29(3):243-59. doi: 10.1385/MN:29:3:243. Mol Neurobiol. 2004. PMID: 15181237 Review.
-
Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits.Pharmacol Biochem Behav. 2021 Jan;200:173072. doi: 10.1016/j.pbb.2020.173072. Epub 2020 Nov 20. Pharmacol Biochem Behav. 2021. PMID: 33227308 Free PMC article. Review.
Cited by
-
Reward and aversion in a heterogeneous midbrain dopamine system.Neuropharmacology. 2014 Jan;76 Pt B(0 0):351-9. doi: 10.1016/j.neuropharm.2013.03.019. Epub 2013 Apr 8. Neuropharmacology. 2014. PMID: 23578393 Free PMC article. Review.
-
What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders.Neurobiol Stress. 2018 Jun 2;9:271-285. doi: 10.1016/j.ynstr.2018.05.004. eCollection 2018 Nov. Neurobiol Stress. 2018. PMID: 30450391 Free PMC article. Review.
-
The power of price compels you: Behavioral economic insights into dopamine-based valuation of rewarding and aversively motivated behavior.Brain Res. 2019 Jun 15;1713:32-41. doi: 10.1016/j.brainres.2018.11.043. Epub 2018 Dec 11. Brain Res. 2019. PMID: 30543771 Free PMC article. Review.
-
The heterogeneity of ventral tegmental area neurons: Projection functions in a mood-related context.Neuroscience. 2014 Dec 12;282:101-8. doi: 10.1016/j.neuroscience.2014.06.006. Epub 2014 Jun 12. Neuroscience. 2014. PMID: 24931766 Free PMC article. Review.
-
Brain opioid segments and striatal patterns of dopamine release induced by naloxone and morphine.Hum Brain Mapp. 2022 Mar;43(4):1419-1430. doi: 10.1002/hbm.25733. Epub 2021 Dec 7. Hum Brain Mapp. 2022. PMID: 34873784 Free PMC article.
References
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS (1993) Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther 264: 489–495. - PubMed
-
- Bozarth MA, Wise RA (1984) Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science 224: 516–517. - PubMed
-
- Welzl H, Kuhn G, Huston JP (1989) Self-administration of small amounts of morphine through glass micropipettes into the ventral tegmental area of the rat. Neuropharmacology 28: 1017–1023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

