Identification and characterization of argonaute protein, Ago2 and its associated small RNAs in Schistosoma japonicum
- PMID: 22860145
- PMCID: PMC3409120
- DOI: 10.1371/journal.pntd.0001745
Identification and characterization of argonaute protein, Ago2 and its associated small RNAs in Schistosoma japonicum
Abstract
Background: The complex life cycle of the genus Schistosoma drives the parasites to employ subtle developmentally dependent gene regulatory machineries. Small non-coding RNAs (sncRNAs) are essential gene regulatory factors that, through their impact on mRNA and genome stability, control stage-specific gene expression. Abundant sncRNAs have been identified in this genus. However, their functionally associated partners, Argonaute family proteins, which are the key components of the RNA-induced silencing complex (RISC), have not yet been fully explored.
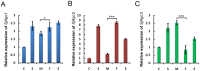
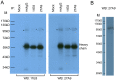
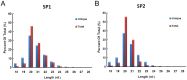
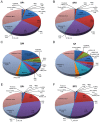
Methodology/principal findings: Two monoclonal antibodies (mAbs) specific to Schistosoma japonicum Argonaute protein Ago2 (SjAgo2), but not SjAgo1 and SjAgo3, were generated. Soluble adult worm antigen preparation (SWAP) was subjected to immunoprecipitation with the mAbs and the captured SjAgo2 protein was subsequently confirmed by Western blot and mass spectrometry (MS) analysis. The small RNA population associated with native SjAgo2 in adult parasites was extracted from the immunoprecipitated complex and subjected to library construction. High-through-put sequencing of these libraries yielded a total of ≈50 million high-quality reads. Classification of these small RNAs showed that endogenous siRNAs (endo-siRNAs) generated from transposable elements (TEs), especially from the subclasses of LINE and LTR, were prominent. Further bioinformatics analysis revealed that siRNAs derived from ten types of well-defined retrotransposons were dramatically enriched in the SjAgo2-specific libraries compared to small RNA libraries constructed with total small RNAs from separated adult worms. These results suggest that a key function of SjAgo2 is to maintain genome stability through suppressing the activities of retrotransposons.
Conclusions/significance: In this study, we identified and characterized one of the three S. japonicum Argonautes, SjAgo2, and its associated small RNAs were found to be predominantly derived from particular classes of retrotransposons. Thus, a major function of SjAgo2 appears to associate with the maintenance of genome stability via suppression of retroelements. The data advance our understanding of the gene regulatory mechanisms in the blood fluke.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Blanchard TJ (2004) Schistosomiasis. Travel Med Infect Dis 2: 5–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

