Label-free visualization of ultrastructural features of artificial synapses via cryo-EM
- PMID: 22860164
- PMCID: PMC3369721
- DOI: 10.1021/cn200094j
Label-free visualization of ultrastructural features of artificial synapses via cryo-EM
Abstract
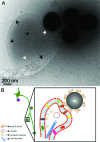
The ultrastructural details of presynapses formed between artificial substrates of submicrometer silica beads and hippocampal neurons are visualized via cryo-electron microscopy (cryo-EM). The silica beads are derivatized by poly-d-lysine or lipid bilayers. Molecular features known to exist at presynapses are clearly present at these artificial synapses, as visualized by cryo-EM. Key synaptic features such as the membrane contact area at synaptic junctions, the presynaptic bouton containing presynaptic vesicles, as well as microtubular structures can be identified. This is the first report of the direct, label-free observation of ultrastructural details of artificial synapses.
Keywords: Artificial synapses; cryo-EM; electron microscopy; hippocampal neurons; synaptic vesicles.
Figures



References
-
- Cowan W. M., Südhof T. C., and Stevens C. F., Eds. (2001) Synapses, Johns Hopkins University Press: Baltimore.
- Dustin M. L.; Colman D. R. (2002) Neural and Immunological Synaptic Relations. Science 298, 785–789. - PubMed
- Bury L. A. D.; Sabo S. L. (2010) Coordinated trafficking of synaptic vesicle and active zone proteins prior to synapse formation. Mol. Interventions 10, 282–292. - PMC - PubMed
-
- Agnati L. F.; Zoli M.; Stromberg I.; Fuxe K. (1995) Intercellular communication in the brain: wiring versus volume transmission. Neuroscience 69, 711–726. - PubMed
- Clemens J. D. (1996) Transmitter timecourse in the synaptic cleft: Its role in central synaptic function. Trends Neurosci. 19, 163–171. - PubMed
- Savtchenko L. P.; Rusakov D. A. (2007) The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. U.S.A. 104, 1823–1828. - PMC - PubMed
-
- Fletcher T. L.; Decamilli P.; Banker G. J. (1994) Synaptogenesis in hippocampal cultures: evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. Neurosci. 14, 6695–6706. - PMC - PubMed
- Jontes J. D.; Buchanan J.; Smith S. J. (2000) Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci. 3, 231–237. - PubMed
- Fuger P.; Behrends L. B.; Mertel S.; Sigrist S. J.; Rasse T. M. (2007) Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nat. Protoc. 2, 3285–3298. - PubMed
- Di Biase V.; Obermair G. J.; Flucher B. E. (2009) Resolving sub-synaptic compartments with double immunofluorescence labeling in hippocampal neurons. J. Neurosci. Methods 176, 78–84. - PubMed
-
- Rostaing P.; Real E.; Siksou L.; Lechaire J. P.; Boudier T.; Boeckers T. M.; Gertler F.; Gundelfinger E. D.; Triller A.; Marty S. (2006) Analysis of synaptic ultrastructure without fixative using high-pressure freezing and tomography. Eur. J. Neurosci. 24, 3463–3474. - PubMed
- Leitch B. (1992) Ultrastructure of electrical synapses: Review. Electron Microsc. Rev. 5, 311–339. - PubMed
- Staehelin L. (1974) Structure and function of intercel- lular junctions, A. Int. Rev. Cytol. 39, 191–283. - PubMed
-
- Fernandez-Busnadiego R.; Zuber B.; Maurer U. E.; Cyrklaff M.; Baumeister W.; Lucic V. (2010) Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J. Cell Biol. 188, 145–156. - PMC - PubMed
- Lucic V.; Yang T.; Schweikert G.; Forster F.; Baumeister W. (2005) Morphological characterization of molecular complexes present in the synaptic cleft. Structure 13, 423–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

