A simple method for quantifying functional selectivity and agonist bias
- PMID: 22860188
- PMCID: PMC3369801
- DOI: 10.1021/cn200111m
A simple method for quantifying functional selectivity and agonist bias
Abstract
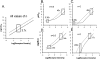
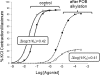
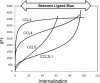
Activation of seven-transmembrane (7TM) receptors by agonists does not always lead to uniform activation of all signaling pathways mediated by a given receptor. Relative to other ligands, many agonists are "biased" toward producing subsets of receptor behaviors. A hallmark of such "functional selectivity" is cell type dependence; this poses a particular problem for the profiling of agonists in whole cell test systems removed from the therapeutic one(s). Such response-specific cell-based variability makes it difficult to guide medicinal chemistry efforts aimed at identifying and optimizing therapeutically meaningful agonist bias. For this reason, we present a scale, based on the Black and Leff operational model, that contains the key elements required to describe 7TM agonism, namely, affinity (K(A) (-1)) for the receptor and efficacy (τ) in activating a particular signaling pathway. Utilizing a "transduction coefficient" term, log(τ/K(A)), this scale can statistically evaluate selective agonist effects in a manner that can theoretically inform structure-activity studies and/or drug candidate selection matrices. The bias of four chemokines for CCR5-mediated inositol phosphate production versus internalization is quantified to illustrate the practical application of this method. The independence of this method with respect to receptor density and the calculation of statistical estimates of confidence of differences are specifically discussed.
Keywords: Biased agonism; drug discovery; functional selectivity; receptor methods; receptor theory; stimulus bias.
Figures

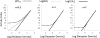

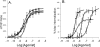

References
-
- Furchgott R. F. (1966) The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. In Advances in Drug Research (Harper N. J., Simmonds A. B., Eds.) Vol. 3, pp 21–55, Academic Press, London.
-
- Kenakin T. P. (2009) A Pharmacology Primer: Theory, Application and Methods, 3rd ed., Academic Press/Elsevier, Amsterdam.
-
- Roth B. L.; Chuang D.-M. (1987) Multiple mechanisms of serotonergic signal transduction. Life Sci. 41, 1051–1064. - PubMed
-
- Lawler C. P.; Prioleua C.; Lewis M. M.; Mak C.; Jiang D.; Schetz J. A.; Gonzalez A. M.; Sibley D. R.; Mailman R. B. (1999) Interactions of the Novel Antipsychotic Aripiprazole (OPC-14597) with Dopamine and Serotonin Receptor Subtypes. Neuropsychopharmacology 20, 612–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

