Potential of a γ-glutamyl-transpeptidase-stable glutathione analogue against amyloid-β toxicity
- PMID: 22860189
- PMCID: PMC3369799
- DOI: 10.1021/cn200113z
Potential of a γ-glutamyl-transpeptidase-stable glutathione analogue against amyloid-β toxicity
Abstract
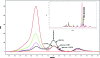
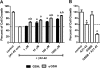
The antioxidant properties of glutathione (GSH) and their relevance to oxidative stress induced pathological states such as Alzheimer's disease is well-established. The utility of GSH itself as a pharmacotherapeutic agent for such disorders is limited because of the former's lability to breakdown through amide cleavage by the ubiquitous enzyme γ-glutamyl transpeptidase (γ-GT). In the present study, a GSH analogue, Ψ-GSH, where the γ-glutamylcysteine amide linkage is replaced with a ureide linkage, was synthesized. Ψ-GSH was found to be stable toward γ-GT mediated breakdown. Ψ-GSH fulfilled four cardinal properties of GSH, namely, traversing across the blood brain barrier (BBB) via the GSH active uptake machinery, replacing GSH in the glyoxalase-I mediated detoxification of methylglyoxal, protecting cells against chemical oxidative insult, and finally lowering the cytotoxicity of amyloid-β peptide. These results validate Ψ-GSH as a viable metabolically stable replacement for GSH and establish it as a potential preclinical candidate for treatment of oxidative stress mediated pathology.
Keywords: Alzheimer’s disease; Glutathione; advanced glycation end products; glyoxalase I; methylglyoxal; β-amyloid peptide.
Figures



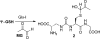


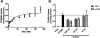
References
-
- Alzheimer’s Association. (2010) Alzheimer’s disease facts and figures. Retrieved, February 18, 2011, from http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf.
-
- Khachaturian Z. S. (1985) Diagnosis of Alzheimer’s disease. Arch. Neurol. 42, 1097–1105. - PubMed
-
- Markesbery W. R. (1997) Oxidative stress hypothesis and Alzheimer’s disease. Free Radical Biol. Med. 23, 134–147. - PubMed
-
- Monnier V. M.; Cerami A. (1981) Nonenzymatic browning in vivo: possible process for aging of long lived proteins. Science 30, 491–493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

