Endogenous molecules stimulating N-acylethanolamine-hydrolyzing acid amidase (NAAA)
- PMID: 22860206
- PMCID: PMC3382453
- DOI: 10.1021/cn300007s
Endogenous molecules stimulating N-acylethanolamine-hydrolyzing acid amidase (NAAA)
Abstract
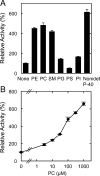
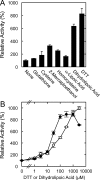
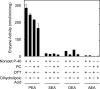
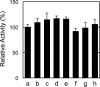
Fatty acid amide hydrolase (FAAH) plays the central role in the degradation of bioactive N-acylethanolamines such as the endocannabinoid arachidonoylethanolamide (anandamide) in brain and peripheral tissues. A lysosomal enzyme referred to as N-acylethanolamine-hydrolyzing acid amidase (NAAA) catalyzes the same reaction with preference to palmitoylethanolamide, an endogenous analgesic and neuroprotective substance, and is therefore expected as a potential target of therapeutic drugs. In the in vitro assays thus far performed, the maximal activity of NAAA was achieved in the presence of both nonionic detergent (Triton X-100 or Nonidet P-40) and the SH reagent dithiothreitol. However, endogenous molecules that might substitute for these synthetic compounds remain poorly understood. Here, we examined stimulatory effects of endogenous phospholipids and thiol compounds on recombinant NAAA. Among different phospholipids tested, choline- or ethanolamine-containing phospholipids showed potent effects, and 1 mM phosphatidylcholine increased NAAA activity by 6.6-fold. Concerning endogenous thiol compounds, dihydrolipoic acid at 0.1-1 mM was the most active, causing 8.5-9.0-fold stimulation. These results suggest that endogenous phospholipids and dihydrolipoic acid may contribute in keeping NAAA active in lysosomes. Even in the presence of phosphatidylcholine and dihydrolipoic acid, however, the preferential hydrolysis of palmitoylethanolamide was unaltered. We also investigated a possible compensatory induction of NAAA mRNA in brain and other tissues of FAAH-deficient mice. However, NAAA expression levels in all the tissues examined were not significantly altered from those in wild-type mice.
Keywords: N-Acylethanolamine-hydrolyzing acid amidase; NAAA; dihydrolipoic acid; fatty acid amide hydrolase; palmitoylethanolamide; phospholipid.
Figures

Similar articles
-
Involvement of N-acylethanolamine-hydrolyzing acid amidase in the degradation of anandamide and other N-acylethanolamines in macrophages.Biochim Biophys Acta. 2005 Oct 1;1736(3):211-20. doi: 10.1016/j.bbalip.2005.08.010. Epub 2005 Aug 29. Biochim Biophys Acta. 2005. PMID: 16154384
-
The N-acylethanolamine-hydrolyzing acid amidase (NAAA).Chem Biodivers. 2007 Aug;4(8):1914-25. doi: 10.1002/cbdv.200790159. Chem Biodivers. 2007. PMID: 17712833 Review.
-
Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase.J Biol Chem. 2005 Mar 25;280(12):11082-92. doi: 10.1074/jbc.M413473200. Epub 2005 Jan 17. J Biol Chem. 2005. PMID: 15655246
-
Predominant expression of lysosomal N-acylethanolamine-hydrolyzing acid amidase in macrophages revealed by immunochemical studies.Biochim Biophys Acta. 2007 May;1771(5):623-32. doi: 10.1016/j.bbalip.2007.03.005. Epub 2007 Mar 24. Biochim Biophys Acta. 2007. PMID: 17462942
-
N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA).Prog Lipid Res. 2010 Oct;49(4):299-315. doi: 10.1016/j.plipres.2010.02.003. Epub 2010 Feb 10. Prog Lipid Res. 2010. PMID: 20152858 Review.
Cited by
-
The Human Ntn-Hydrolase Superfamily: Structure, Functions and Perspectives.Cells. 2022 May 10;11(10):1592. doi: 10.3390/cells11101592. Cells. 2022. PMID: 35626629 Free PMC article. Review.
-
2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation.Front Pharmacol. 2017 May 30;8:308. doi: 10.3389/fphar.2017.00308. eCollection 2017. Front Pharmacol. 2017. PMID: 28611664 Free PMC article.
-
The rise and fall of anandamide: processes that control synthesis, degradation, and storage.Mol Cell Biochem. 2021 Jul;476(7):2753-2775. doi: 10.1007/s11010-021-04121-5. Epub 2021 Mar 13. Mol Cell Biochem. 2021. PMID: 33713246 Review.
-
Inhibition of triple negative breast cancer-associated inflammation and progression by N- acylethanolamine acid amide hydrolase (NAAA).Sci Rep. 2022 Dec 23;12(1):22255. doi: 10.1038/s41598-022-26564-6. Sci Rep. 2022. PMID: 36564457 Free PMC article.
-
Molecular mechanism of activation of the immunoregulatory amidase NAAA.Proc Natl Acad Sci U S A. 2018 Oct 23;115(43):E10032-E10040. doi: 10.1073/pnas.1811759115. Epub 2018 Oct 9. Proc Natl Acad Sci U S A. 2018. PMID: 30301806 Free PMC article.
References
-
- Schmid H. H. O.; Schmid P. C.; Natarajan V. (1990) N-Acylated glycerophospholipids and their derivatives. Prog. Lipid Res. 29, 1–43. - PubMed
-
- Devane W. A.; Hanuš L.; Breuer A.; Pertwee R. G.; Stevenson L. A.; Griffin G.; Gibson D.; Mandelbaum A.; Etinger A.; Mechoulam R. (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949. - PubMed
-
- Zygmunt P. M.; Petersson J.; Andersson D. A.; Chuang H.; Sørgård M.; Di Marzo V.; Julius D.; Högestätt E. D. (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400, 452–457. - PubMed
-
- Pertwee R. G.; Howlett A. C.; Abood M. E.; Alexander S. P. H; Di Marzo V.; Elphick M. R.; Greasley P. J.; Hansen H. S.; Kunos G.; Mackie K.; Mechoulam R.; Ross R. A. (2010) International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 62, 588–631. - PMC - PubMed
-
- Calignano A.; La Rana G.; Giuffrida A.; Piomelli D. (1998) Control of pain initiation by endogenous cannabinoids. Nature 394, 277–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

