O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors
- PMID: 22860211
- PMCID: PMC3382460
- DOI: 10.1021/cn200089j
O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors
Abstract
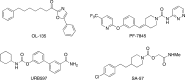
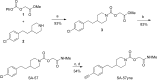
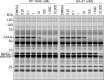
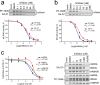
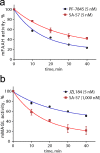
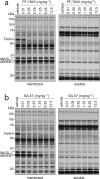
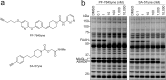
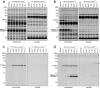
The two major endocannabinoid transmitters, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), are degraded by distinct enzymes in the nervous system, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. FAAH and MAGL inhibitors cause elevations in brain AEA and 2-AG levels, respectively, and reduce pain, anxiety, and depression in rodents without causing the full spectrum of psychotropic behavioral effects observed with direct cannabinoid receptor-1 (CB1) agonists. These findings have inspired the development of several classes of endocannabinoid hydrolase inhibitors, most of which have been optimized to show specificity for either FAAH or MAGL or, in certain cases, equipotent activity for both enzymes. Here, we investigate an unusual class of O-hydroxyacetamide carbamate inhibitors and find that individual compounds from this class can serve as selective FAAH or dual FAAH/MAGL inhibitors in vivo across a dose range (0.125-12.5 mg kg(-1)) suitable for behavioral studies. Competitive and click chemistry activity-based protein profiling confirmed that the O-hydroxyacetamide carbamate SA-57 is remarkably selective for FAAH and MAGL in vivo, targeting only one other enzyme in brain, the additional 2-AG hydrolase ABHD6. These data designate O-hydroxyacetamide carbamates as a versatile chemotype for creating endocannabinoid hydrolase inhibitors that display excellent in vivo activity and tunable selectivity for FAAH-anandamide versus MAGL (and ABHD6)-2-AG pathways.
Keywords: 2-arachidonoylglycerol; Activity-based protein profiling; anandamide; carbamate; endocannabinoid; hydrolase.
Figures
Similar articles
-
Inhibition of the endocannabinoid-regulating enzyme monoacylglycerol lipase elicits a CB1 receptor-mediated discriminative stimulus in mice.Neuropharmacology. 2017 Oct;125:80-86. doi: 10.1016/j.neuropharm.2017.06.032. Epub 2017 Jun 30. Neuropharmacology. 2017. PMID: 28673548 Free PMC article.
-
Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates.Chem Biol. 2012 May 25;19(5):579-88. doi: 10.1016/j.chembiol.2012.03.009. Epub 2012 Apr 26. Chem Biol. 2012. PMID: 22542104 Free PMC article.
-
Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo.Proc Natl Acad Sci U S A. 2009 Dec 1;106(48):20270-5. doi: 10.1073/pnas.0909411106. Epub 2009 Nov 16. Proc Natl Acad Sci U S A. 2009. PMID: 19918051 Free PMC article.
-
Overview of the chemical families of fatty acid amide hydrolase and monoacylglycerol lipase inhibitors.Curr Top Med Chem. 2008;8(3):247-67. doi: 10.2174/156802608783498005. Curr Top Med Chem. 2008. PMID: 18289091 Review.
-
Inhibitors of the endocannabinoid-degrading enzymes, or how to increase endocannabinoid's activity by preventing their hydrolysis.Recent Pat CNS Drug Discov. 2012 Apr 1;7(1):49-70. doi: 10.2174/157488912798842223. Recent Pat CNS Drug Discov. 2012. PMID: 22280341 Review.
Cited by
-
Integrated phenotypic and activity-based profiling links Ces3 to obesity and diabetes.Nat Chem Biol. 2014 Feb;10(2):113-21. doi: 10.1038/nchembio.1429. Epub 2013 Dec 22. Nat Chem Biol. 2014. PMID: 24362705 Free PMC article.
-
The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice.Neuropharmacology. 2017 Mar 1;114:156-167. doi: 10.1016/j.neuropharm.2016.11.015. Epub 2016 Nov 25. Neuropharmacology. 2017. PMID: 27890602 Free PMC article.
-
FAAH Modulators from Natural Sources: A Collection of New Potential Drugs.Cells. 2025 Apr 5;14(7):551. doi: 10.3390/cells14070551. Cells. 2025. PMID: 40214504 Free PMC article. Review.
-
A protocol to visualize on-target specific drug binding in mammalian tissue with cellular resolution using tissue clearing and click chemistry.STAR Protoc. 2022 Oct 25;3(4):101778. doi: 10.1016/j.xpro.2022.101778. eCollection 2022 Dec 16. STAR Protoc. 2022. PMID: 36313539 Free PMC article.
-
Intracranial self-stimulation to evaluate abuse potential of drugs.Pharmacol Rev. 2014 Jul;66(3):869-917. doi: 10.1124/pr.112.007419. Pharmacol Rev. 2014. PMID: 24973197 Free PMC article. Review.
References
-
- Ligresti A.; Petrosino S.; Di Marzo V. (2009) From endocannabinoid profiling to ’endocannabinoid therapeutics’. Curr. Opin. Chem. Biol. 13, 321–331. - PubMed
-
- Di Marzo V. (2008) Targeting the endocannabinoid system: to enhance or reduce?. Nat. Rev. Drug Discovery 7, 438–455. - PubMed
-
- Fowler C. J. (2008) ”The tools of the trade”--an overview of the pharmacology of the endocannabinoid system. Curr. Pharm. Des. 14, 2254–2265. - PubMed
-
- Graham E. S.; Ashton J. C.; Glass M. (2009) Cannabinoid receptors: A brief history and “what’s hot”. Front. Biosci. 14, 944–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

