O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors
- PMID: 22860211
- PMCID: PMC3382460
- DOI: 10.1021/cn200089j
O-hydroxyacetamide carbamates as a highly potent and selective class of endocannabinoid hydrolase inhibitors
Abstract
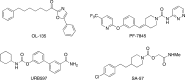
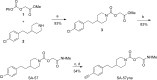
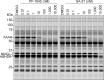
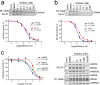
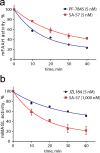
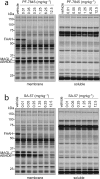
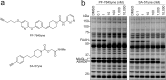
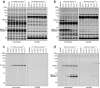
The two major endocannabinoid transmitters, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), are degraded by distinct enzymes in the nervous system, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. FAAH and MAGL inhibitors cause elevations in brain AEA and 2-AG levels, respectively, and reduce pain, anxiety, and depression in rodents without causing the full spectrum of psychotropic behavioral effects observed with direct cannabinoid receptor-1 (CB1) agonists. These findings have inspired the development of several classes of endocannabinoid hydrolase inhibitors, most of which have been optimized to show specificity for either FAAH or MAGL or, in certain cases, equipotent activity for both enzymes. Here, we investigate an unusual class of O-hydroxyacetamide carbamate inhibitors and find that individual compounds from this class can serve as selective FAAH or dual FAAH/MAGL inhibitors in vivo across a dose range (0.125-12.5 mg kg(-1)) suitable for behavioral studies. Competitive and click chemistry activity-based protein profiling confirmed that the O-hydroxyacetamide carbamate SA-57 is remarkably selective for FAAH and MAGL in vivo, targeting only one other enzyme in brain, the additional 2-AG hydrolase ABHD6. These data designate O-hydroxyacetamide carbamates as a versatile chemotype for creating endocannabinoid hydrolase inhibitors that display excellent in vivo activity and tunable selectivity for FAAH-anandamide versus MAGL (and ABHD6)-2-AG pathways.
Keywords: 2-arachidonoylglycerol; Activity-based protein profiling; anandamide; carbamate; endocannabinoid; hydrolase.
Figures
References
-
- Ligresti A.; Petrosino S.; Di Marzo V. (2009) From endocannabinoid profiling to ’endocannabinoid therapeutics’. Curr. Opin. Chem. Biol. 13, 321–331. - PubMed
-
- Di Marzo V. (2008) Targeting the endocannabinoid system: to enhance or reduce?. Nat. Rev. Drug Discovery 7, 438–455. - PubMed
-
- Fowler C. J. (2008) ”The tools of the trade”--an overview of the pharmacology of the endocannabinoid system. Curr. Pharm. Des. 14, 2254–2265. - PubMed
-
- Graham E. S.; Ashton J. C.; Glass M. (2009) Cannabinoid receptors: A brief history and “what’s hot”. Front. Biosci. 14, 944–957. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

