The pig as a model for investigating the role of neutrophil serine proteases in human inflammatory lung diseases
- PMID: 22860995
- PMCID: PMC3492928
- DOI: 10.1042/BJ20120818
The pig as a model for investigating the role of neutrophil serine proteases in human inflammatory lung diseases
Abstract
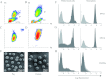
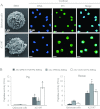
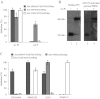
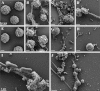
The serine proteases released by activated polymorphonuclear neutrophils [NSPs (neutrophil serine proteases)] contribute to a variety of inflammatory lung diseases, including CF (cystic fibrosis). They are therefore key targets for the development of efficient inhibitors. Although rodent models have contributed to our understanding of several diseases, we have previously shown that they are not appropriate for testing anti-NSP therapeutic strategies [Kalupov, Brillard-Bourdet, Dade, Serrano, Wartelle, Guyot, Juliano, Moreau, Belaaouaj and Gauthier (2009) J. Biol. Chem. 284, 34084-34091). Thus NSPs must be characterized in an animal model that is much more likely to predict how therapies will act in humans in order to develop protease inhibitors as drugs. The recently developed CFTR-/- (CFTR is CF transmembrane conductance regulator) pig model is a promising alternative to the mouse model of CF [Rogers, Stoltz, Meyerholz, Ostedgaard, Rokhlina, Taft, Rogan, Pezzulo, Karp, Itani et al. (2008) Science 321, 1837-1841]. We have isolated blood neutrophils from healthy pigs and determined their responses to the bacterial pathogens Pseudomonas aeruginosa and Staphylococcus aureus, and the biochemical properties of their NSPs. We used confocal microscopy and antibodies directed against their human homologues to show that the three NSPs (elastase, protease 3 and cathepsin G) are enzymatically active and present on the surface of triggered neutrophils and NETs (neutrophil extracellular traps). All of the porcine NSPs are effectively inhibited by human NSP inhibitors. We conclude that there is a close functional resemblance between porcine and human NSPs. The pig is therefore a suitable animal model for testing new NSP inhibitors as anti-inflammatory agents in neutrophil-associated diseases such as CF.
Figures
Similar articles
-
The Pig: A Relevant Model for Evaluating the Neutrophil Serine Protease Activities during Acute Pseudomonas aeruginosa Lung Infection.PLoS One. 2016 Dec 16;11(12):e0168577. doi: 10.1371/journal.pone.0168577. eCollection 2016. PLoS One. 2016. PMID: 27992534 Free PMC article.
-
Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum.Am J Respir Cell Mol Biol. 2012 Jul;47(1):80-6. doi: 10.1165/rcmb.2011-0380OC. Epub 2012 Feb 16. Am J Respir Cell Mol Biol. 2012. PMID: 22343221
-
Neutrophil serine proteases in cystic fibrosis: role in disease pathogenesis and rationale as a therapeutic target.Eur Respir Rev. 2024 Sep 18;33(173):240001. doi: 10.1183/16000617.0001-2024. Print 2024 Jul. Eur Respir Rev. 2024. PMID: 39293854 Free PMC article. Review.
-
Pharmacological inhibition of cathepsin S and of NSPs-AAP-1 (a novel, alternative protease driving the activation of neutrophil serine proteases).Biochem Pharmacol. 2024 Nov;229:116114. doi: 10.1016/j.bcp.2024.116114. Epub 2024 Sep 16. Biochem Pharmacol. 2024. PMID: 39455238
-
Cathepsin C inhibition as a potential treatment strategy in cancer.Biochem Pharmacol. 2021 Dec;194:114803. doi: 10.1016/j.bcp.2021.114803. Epub 2021 Oct 20. Biochem Pharmacol. 2021. PMID: 34678221 Review.
Cited by
-
The Pig: A Relevant Model for Evaluating the Neutrophil Serine Protease Activities during Acute Pseudomonas aeruginosa Lung Infection.PLoS One. 2016 Dec 16;11(12):e0168577. doi: 10.1371/journal.pone.0168577. eCollection 2016. PLoS One. 2016. PMID: 27992534 Free PMC article.
-
Shift of Neutrophils From Blood to Bone Marrow Upon Extensive Experimental Trauma Surgery.Front Immunol. 2022 May 17;13:883863. doi: 10.3389/fimmu.2022.883863. eCollection 2022. Front Immunol. 2022. PMID: 35655784 Free PMC article.
-
Global substrate profiling of proteases in human neutrophil extracellular traps reveals consensus motif predominantly contributed by elastase.PLoS One. 2013 Sep 20;8(9):e75141. doi: 10.1371/journal.pone.0075141. eCollection 2013. PLoS One. 2013. PMID: 24073241 Free PMC article.
-
Computed Tomography (CT) Scanning Facilitates Early Identification of Neonatal Cystic Fibrosis Piglets.PLoS One. 2015 Nov 23;10(11):e0143459. doi: 10.1371/journal.pone.0143459. eCollection 2015. PLoS One. 2015. PMID: 26600426 Free PMC article.
-
The porcine innate immune system: an update.Dev Comp Immunol. 2014 Aug;45(2):321-43. doi: 10.1016/j.dci.2014.03.022. Epub 2014 Apr 4. Dev Comp Immunol. 2014. PMID: 24709051 Free PMC article. Review.
References
-
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
-
- Papayannopoulos V., Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol. 2009;30:513–521. - PubMed
-
- Stockley R. A. Neutrophils and protease/antiprotease imbalance. Am. J. Respir. Crit. Care Med. 1999;160:S49–S52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

