DNA charge transport for sensing and signaling
- PMID: 22861008
- PMCID: PMC3495616
- DOI: 10.1021/ar3001298
DNA charge transport for sensing and signaling
Abstract
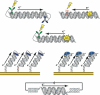
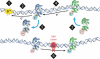
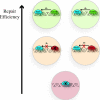
The DNA duplex is an exquisite macromolecular array that stores genetic information to encode proteins and regulate pathways. Its unique structure also imparts chemical function that allows it also to mediate charge transport (CT). We have utilized diverse platforms to probe DNA CT, using spectroscopic, electrochemical, and even genetic methods. These studies have established powerful features of DNA CT chemistry. DNA CT can occur over long molecular distances as long as the bases are well stacked. The perturbations in base stacking that arise with single base mismatches, DNA lesions, and the binding of some proteins that kink the DNA all inhibit DNA CT. Significantly, single molecule studies of DNA CT show that ground state CT can occur over 34 nm if the duplex is well stacked; one single base mismatch inhibits CT. The DNA duplex is an effective sensor for the integrity of the base pair stack. Moreover, the efficiency of DNA CT is what one would expect for a stack of graphite sheets: equivalent to the stack of DNA base pairs and independent of the sugar-phosphate backbone. Since DNA CT offers a means to carry out redox chemistry from a distance, we have considered how this chemistry might be used for long range biological signaling. We have taken advantage of our chemical probes and platforms to characterize DNA CT in the context of the cell. CT can occur over long distances, perhaps funneling damage to particular sites and insulating others from oxidative stress. Significantly, transcription factors that activate the genome to respond to oxidative stress can also be activated from a distance through DNA CT. Numerous proteins maintain the integrity of the genome and an increasing number of them contain [4Fe-4S] clusters that do not appear to carry out either structural or enzymatic roles. Using electrochemical methods, we find that DNA binding shifts the redox potentials of the clusters, activating them towards oxidation at physiological potentials. We have proposed a model that describes how repair proteins may utilize DNA CT to efficiently search the genome for lesions. Importantly, many of these proteins occur in low copy numbers within the cell, and thus a processive mechanism does not provide a sufficient explanation of how they find and repair lesions before the cell divides. Using atomic force microscopy and genetic assays, we show that repair proteins proficient at DNA CT can relocalize in the vicinity of DNA lesions and can cooperate in finding lesions within the cell. Conversely, proteins defective in DNA CT cannot relocalize in the vicinity of lesions and do not assist other proteins involved in repair within the cell. Moreover such genetic defects are associated with disease in human protein analogues. As we continue to unravel this chemistry and discover more proteins with redox cofactors involved in genome maintenance, we are learning more regarding opportunities for long range signaling and sensing, and more examples of DNA CT chemistry that may provide critical functions within the cell.
Figures




References
-
- Eley DD, Spivey DI. Semiconductivity of organic substances. Part 9. Nucleic acid in the dry state. Trans. Faraday Soc. 1962;58:411–415.
-
- Wagenknecht HA, editor. Charge Transfer in DNA: From Mechanism to Application. Wiley-VCH, Weinheim; Germany: 2005.
-
- Kelley SO, Barton JK. Electron Transfer Between Bases in Double Helical DNA. Science. 1999;283:375–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

