How anti-neutrophil cytoplasmic autoantibodies activate neutrophils
- PMID: 22861361
- PMCID: PMC3444998
- DOI: 10.1111/j.1365-2249.2012.04615.x
How anti-neutrophil cytoplasmic autoantibodies activate neutrophils
Abstract
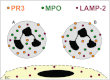
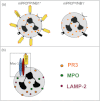
Neutrophils are pivotal to host defence during infectious diseases. However, activated neutrophils may also cause undesired tissue damage. Ample examples include small-vessel inflammatory diseases (vasculitis) that are associated with anti-neutrophil cytoplasmic autoantibodies (ANCA) residing in the patients' plasma. In addition to being an important diagnostic tool, convincing evidence shows that ANCA are pathogenic. ANCA-neutrophil interactions induce important cellular responses that result in highly inflammatory necrotizing vascular damage. The interaction begins with ANCA binding to their target antigens on primed neutrophils, proceeds by recruiting transmembrane molecules to initiate intracellular signal transduction and culminates in activation of effector functions that ultimately mediate the tissue damage.
© 2012 The Author. Clinical and Experimental Immunology © 2012 British Society for Immunology.
Figures

References
-
- Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–65. - PubMed
-
- Sengelov H, Kjeldsen L, Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–43. - PubMed
-
- Witko Sarsat V, Cramer EM, Hieblot C, et al. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood. 1999;94:2487–96. - PubMed
-
- Charles LA, Caldas ML, Falk RJ, Terrell RS, Jennette JC. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991;50:539–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

