TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends
- PMID: 22863003
- PMCID: PMC3516183
- DOI: 10.1016/j.cell.2012.07.012
TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends
Abstract
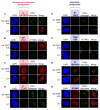
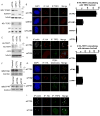
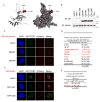
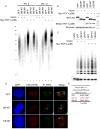
Telomere synthesis in cancer cells and stem cells involves trafficking of telomerase to Cajal bodies, and telomerase is thought to be recruited to telomeres through interactions with telomere-binding proteins. Here, we show that the OB-fold domain of the telomere-binding protein TPP1 recruits telomerase to telomeres through an association with the telomerase reverse transcriptase TERT. When tethered away from telomeres and other telomere-binding proteins, the TPP1 OB-fold domain is sufficient to recruit telomerase to a heterologous chromatin locus. Expression of a minimal TPP1 OB-fold inhibits telomere maintenance by blocking access of telomerase to its cognate binding site at telomeres. We identify amino acids required for the TPP1-telomerase interaction, including specific loop residues within the TPP1 OB-fold domain and individual residues within TERT, some of which are mutated in a subset of pulmonary fibrosis patients. These data define a potential interface for telomerase-TPP1 interaction required for telomere maintenance and implicate defective telomerase recruitment in telomerase-related disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Exposing secrets of telomere-telomerase encounters.Cell. 2012 Aug 3;150(3):453-4. doi: 10.1016/j.cell.2012.07.015. Cell. 2012. PMID: 22863000
References
-
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, 3rd, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

