Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ
- PMID: 22863012
- PMCID: PMC3413172
- DOI: 10.1016/j.cell.2012.06.027
Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ
Abstract
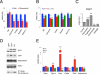
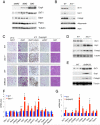
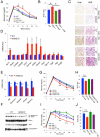
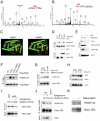
Brown adipose tissue (BAT) can disperse stored energy as heat. Promoting BAT-like features in white adipose (WAT) is an attractive, if elusive, therapeutic approach to staunch the current obesity epidemic. Here we report that gain of function of the NAD-dependent deacetylase SirT1 or loss of function of its endogenous inhibitor Deleted in breast cancer-1 (Dbc1) promote "browning" of WAT by deacetylating peroxisome proliferator-activated receptor (Ppar)-γ on Lys268 and Lys293. SirT1-dependent deacetylation of Lys268 and Lys293 is required to recruit the BAT program coactivator Prdm16 to Pparγ, leading to selective induction of BAT genes and repression of visceral WAT genes associated with insulin resistance. An acetylation-defective Pparγ mutant induces a brown phenotype in white adipocytes, whereas an acetylated mimetic fails to induce "brown" genes but retains the ability to activate "white" genes. We propose that SirT1-dependent Pparγ deacetylation is a form of selective Pparγ modulation of potential therapeutic import.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Comment in
-
PPARγ: no SirT, no service.Circ Res. 2013 Feb 1;112(3):411-4. doi: 10.1161/CIRCRESAHA.113.300870. Circ Res. 2013. PMID: 23371897 Free PMC article.
References
-
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK063608/DK/NIDDK NIH HHS/United States
- UL1 RR024156/RR/NCRR NIH HHS/United States
- HL087123/HL/NHLBI NIH HHS/United States
- R37 DK058282/DK/NIDDK NIH HHS/United States
- R01 DK057539/DK/NIDDK NIH HHS/United States
- P30 DK026687/DK/NIDDK NIH HHS/United States
- P30 DK063608/DK/NIDDK NIH HHS/United States
- R01 DK064773/DK/NIDDK NIH HHS/United States
- R01 DK064819/DK/NIDDK NIH HHS/United States
- R01 DK058282/DK/NIDDK NIH HHS/United States
- DK64773/DK/NIDDK NIH HHS/United States
- RR024156/RR/NCRR NIH HHS/United States
- P01 HL087123/HL/NHLBI NIH HHS/United States
- DK58282/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

