Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells
- PMID: 22863534
- PMCID: PMC4023631
- DOI: 10.1016/j.stem.2012.05.024
Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells
Abstract
Leukemia stem cells (LSCs) represent a biologically distinct subpopulation of myeloid leukemias, with reduced cell cycle activity and increased resistance to therapeutic challenge. To better characterize key properties of LSCs, we employed a strategy based on identification of genes synergistically dysregulated by cooperating oncogenes. We hypothesized that such genes, termed "cooperation response genes" (CRGs), would represent regulators of LSC growth and survival. Using both a primary mouse model and human leukemia specimens, we show that CRGs comprise genes previously undescribed in leukemia pathogenesis in which multiple pathways modulate the biology of LSCs. In addition, our findings demonstrate that the CRG expression profile can be used as a drug discovery tool for identification of compounds that selectively target the LSC population. We conclude that CRG-based analyses provide a powerful means to characterize the basic biology of LSCs as well as to identify improved methods for therapeutic targeting.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


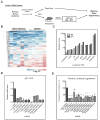

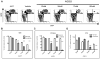
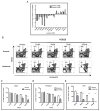
Comment in
-
Modelling how initiating and transforming oncogenes cooperate to produce a leukaemic cell state.Dis Model Mech. 2013 Jan;6(1):3-5. doi: 10.1242/dmm.011288. Dis Model Mech. 2013. PMID: 23268533 Free PMC article.
References
-
- Antalis TM, La Linn M, Donnan K, Mateo L, Gardner J, Dickinson JL, Buttigieg K, Suhrbier A. The serine proteinase inhibitor (serpin) plasminogen activation inhibitor type 2 protects against viral cytopathic effects by constitutive interferon alpha/beta priming. The Journal of experimental medicine. 1998;187:1799–1811. - PMC - PubMed
-
- Benjamini YHY. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Statist Soc B. 1995;57:289–300.
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. NatMed. 1997;3:730–737. - PubMed
-
- Burnett A, Wetzler M, Lowenberg B. Therapeutic Advances in Acute Myeloid Leukemia. J Clin Oncol 2011 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

