Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment
- PMID: 22863621
- PMCID: PMC3428088
- DOI: 10.1172/JCI62749
Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment
Abstract
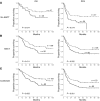
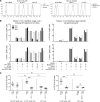
Survival outcomes for patients with high-risk neuroblastoma (NB) have significantly improved with anti-disialoganglioside GD2 mAb therapy, which promotes NK cell activation through antibody-dependent cell-mediated cytotoxicity. NK cell activation requires an interaction between inhibitory killer cell immunoglobulin-like receptors (KIRs) and HLA class I ligands. NK cells lacking KIRs that are specific for self HLA are therefore "unlicensed" and hyporesponsive. mAb-treated NB patients lacking HLA class I ligands for their inhibitory KIRs have significantly higher survival rates, suggesting that NK cells expressing KIRs for non-self HLA are mediating tumor control in these individuals. We found that, in the presence of mAb, both licensed and unlicensed NK cells are highly activated in vitro. However, HLA class I expression on NB cell lines selectively inhibited licensed NK cell activity, permitting primarily unlicensed NK cells to mediate antibody-dependent cell-mediated cytotoxicity. These results indicate that unlicensed NK cells play a key antitumor role in patients undergoing mAb therapy via antibody-dependent cell-mediated cytotoxicity, thus explaining the potent "missing KIR ligand" benefit in patients with NB.
Figures



Comment in
-
When NK cells overcome their lack of education.J Clin Invest. 2012 Sep;122(9):3053-6. doi: 10.1172/JCI63524. Epub 2012 Aug 6. J Clin Invest. 2012. PMID: 22863616 Free PMC article.
-
Immunotherapy: Unlicensed to kill.Nat Rev Cancer. 2012 Oct;12(10):658-9. doi: 10.1038/nrc3359. Epub 2012 Aug 30. Nat Rev Cancer. 2012. PMID: 22932647 No abstract available.
-
Immunotherapy: Unlicensed to kill.Nat Rev Immunol. 2012 Oct;12(10):682-3. doi: 10.1038/nri3301. Epub 2012 Aug 31. Nat Rev Immunol. 2012. PMID: 22941444 No abstract available.
References
-
- Cheung NK, et al. Anti-G(D2) antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16(9):3053–3060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

