Adrenergic nerves govern circadian leukocyte recruitment to tissues
- PMID: 22863835
- PMCID: PMC3428436
- DOI: 10.1016/j.immuni.2012.05.021
Adrenergic nerves govern circadian leukocyte recruitment to tissues
Abstract
The multistep sequence leading to leukocyte migration is thought to be locally regulated at the inflammatory site. Here, we show that broad systemic programs involving long-range signals from the sympathetic nervous system (SNS) delivered by adrenergic nerves regulate rhythmic recruitment of leukocytes in tissues. Constitutive leukocyte adhesion and migration in murine bone marrow (BM) and skeletal-muscle microvasculature fluctuated with circadian peak values at night. Migratory oscillations, altered by experimental jet lag, were implemented by perivascular SNS fibers acting on β-adrenoreceptors expressed on nonhematopoietic cells and leading to tissue-specific, differential circadian oscillations in the expression of endothelial cell adhesion molecules and chemokines. We showed that these rhythms have physiological consequences through alteration of hematopoietic cell recruitment and overall survival in models of septic shock, sickle cell vaso-occlusion, and BM transplantation. These data provide unique insights in the leukocyte adhesion cascade and the potential for time-based therapeutics for transplantation and inflammatory diseases.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

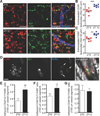
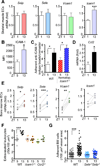


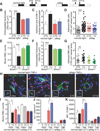

Comment in
-
Regulate globally, act locally: adrenergic nerves promote leukocyte recruitment.Immunity. 2012 Aug 24;37(2):189-91. doi: 10.1016/j.immuni.2012.08.004. Immunity. 2012. PMID: 22921114 Free PMC article.
References
-
- Alito AE, Romeo HE, Baler R, Chuluyan HE, Braun M, Cardinali DP. Autonomic nervous system regulation of murine immune responses as assessed by local surgical sympathetic and parasympathetic denervation. Acta Physiol Pharmacol Latinoam. 1987;37:305–319. - PubMed
-
- Bullard DC, Kunkel EJ, Kubo H, Hicks MJ, Lorenzo I, Doyle NA, Doerschuk CM, Ley K, Beaudet AL. Infectious susceptibility and severe deficiency of leukocyte rolling and recruitment in E-selectin and P-selectin double mutant mice. The Journal of experimental medicine. 1996;183:2329–2336. - PMC - PubMed
-
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

