Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker
- PMID: 22863839
- PMCID: PMC3494841
- DOI: 10.1093/ndt/gfs313
Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker
Abstract
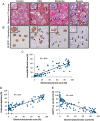
Background: Proteinuria and/or albuminuria are widely used for noninvasive assessment of kidney diseases. However, proteinuria is a nonspecific marker of diverse forms of kidney injury, physiologic processes and filtration of small proteins of monoclonal and other pathologic processes. The opportunity to develop new glomerular disease biomarkers follows the realization that the degree of podocyte depletion determines the degree of glomerulosclerosis, and if persistent, determines the progression to end-stage kidney disease (ESKD). Podocyte cell lineage-specific mRNAs can be recovered in urine pellets of model systems and in humans. In model systems, progressive glomerular disease is associated with decreased nephrin mRNA steady-state levels compared with podocin mRNA. Thus, the urine podocin:nephrin mRNA ratio (PNR) could serve as a useful progression biomarker. The use of podocyte-specific transcript ratios also circumvents many problems inherent to urine assays.
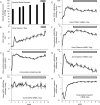
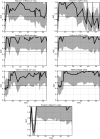
Methods: To test this hypothesis, the human diphtheria toxin receptor (hDTR) rat model of progression was used to evaluate potentially useful urine mRNA biomarkers. We compared histologic progression parameters (glomerulosclerosis score, interstitial fibrosis score and percent of podocyte depletion) with clinical biomarkers [serum creatinine, systolic blood pressure (BP), 24-h urine volume, 24-h urine protein excretion and the urine protein:creatinine ratio(PCR)] and with the novel urine mRNA biomarkers.
Results: The PNR correlated with histologic outcome as well or better than routine clinical biomarkers and other urine mRNA biomarkers in the model system with high specificity and sensitivity, and a low coefficient of assay variation.
Conclusions: We concluded that the PNR, used in combination with proteinuria, will be worth testing for its clinical diagnostic and decision-making utility.
Figures


References
-
- Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech. 2002;57:189–195. - PubMed
-
- Wiggins R. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
-
- Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. - PubMed
-
- Steffes MW, Schmidt D, McCrery R, et al. International diabetic nephropathy study group: glomerular cell number in normal subjects and type I diabetes patients. Kidney Int. 2001;59:2104–2113. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

